通过反应耦合液-液相分离和竞争抑制的可编程凝聚液滴
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由液-液相分离(LLPS)形成的无膜生物分子凝聚体对许多时空生物学功能至关重要。设计合成模拟物来模拟和理解LLPS是一个活跃的研究领域,它通过优雅的生物灵感设计导致了凝聚液滴的发展。然而,最近对这一领域的兴趣已经转向设计可编程凝聚体,以赋予这些液相的时空控制。在此,我们通过采用竞争结合和涉及动态共价键的反应耦合组装的概念,证明了LLPS在合成系统中的编程。我们的研究结果利用了遵循简单凝聚机制的小型构建块,将这种方法与以前报道的可编程复杂凝聚区分开来,后者通常依赖于其中一个组件的反应控制生成。我们使用动态共价键(硼酸酯)和末端附加硼酸基团的小发色基团来介绍这些概念。在与底物(单糖)反应后,这些构建块形成类似于“贴纸和间隔”设计的分子结构,用于凝聚,导致反应驱动,时间控制的LLPS过程。各种单糖的差异反应性,结合动态键的可逆性,使得对生长、抑制和溶解凝聚过程的竞争性结合驱动控制成为可能,为可编程LLPS提供了新的策略,这让人想起生物分子凝聚物中蛋白质诱导的抑制。详细的光谱探测和动力学分析提供了对反应耦合和自催化生长过程的机理见解,揭示了该凝聚系统的葡萄糖选择性性质。最后,将动态共价反应与时间pH调制耦合,产生瞬态共聚焦反应,这一过程可以用共聚焦显微镜观察到。我们预计这种方法将为设计具有新型生物相关涌现特性的凝聚液滴铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
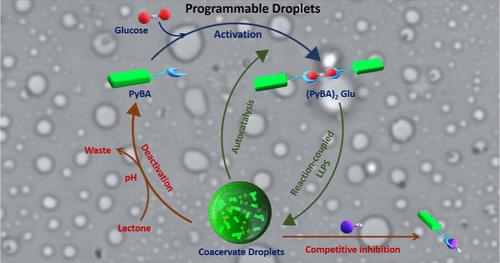
Programmable Coacervate Droplets via Reaction-Coupled Liquid–Liquid Phase Separation (LLPS) and Competitive Inhibition
Membraneless biomolecular condensates formed by liquid–liquid phase separation (LLPS) are crucial for many spatiotemporal biological functions. Designing synthetic mimics to emulate and understand LLPS is an active area of research, which has led to the development of coacervate droplets through elegant bioinspired designs. However, recent interest in this field has shifted toward designing programmable coacervates to impart spatiotemporal control over these liquid phases. Herein, we demonstrate the programming of LLPS in synthetic systems by employing concepts of competitive binding and reaction-coupled assembly involving dynamic covalent bonds. Our results utilize small building blocks that follow a simple coacervation mechanism, distinguishing this approach from previously reported programmable complex coacervates, which often rely on reaction-controlled generation of one of the components. We introduce these concepts using dynamic covalent bonds (boronate esters) and small chromophoric building blocks appended with terminal boronic acid groups. Upon reaction with substrates (monosaccharides), these building blocks form molecular structures resembling “sticker-and-spacer” designs for coacervation, leading to a reaction-driven, temporally controlled LLPS process. The differential reactivity of various monosaccharides, combined with the reversibility of dynamic bonds, enables competitive binding-driven control over the growth, inhibition, and dissolution of the coacervation process, offering new strategies for programmable LLPS that are reminiscent of protein-induced inhibition in biomolecular condensates. Detailed spectroscopic probing and kinetic analyses provide mechanistic insights into the reaction-coupled and autocatalytic growth processes, revealing the glucose-selective nature of this coacervation system. Finally, coupling dynamic covalent reactions with temporal pH modulation results in transient coacervation, which can be visualized by using confocal microscopy. We anticipate that this approach will pave the way for designing coacervate droplets with novel biorelevant emergent properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


