(NHC)Au(I)-H和(NHC)Au(I)-F跨炔反加成反应机理的研究
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-28
DOI:10.1039/d5qo00432b
引用次数: 0
摘要
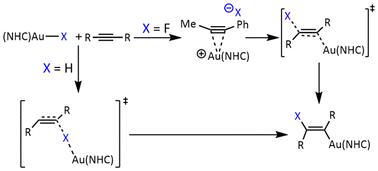
实验观察到(NHC)Au(I)-H与二甲基乙炔二羧酸盐(DMAD) MeOOCC≡coome和(NHC)Au(I)-F与苯乙炔MeC≡CPh的反加成反应是有趣的,值得更深入的研究。本文借助密度泛函理论(DFT)计算和本构键轨道(IBO)分析,系统地研究了(NHC)Au(I)-X (X = H, Me和卤化物)与不同炔烃的加成反应。我们发现两种反加成反应的性质是不同的。(NHC)Au(I)-H的加入是由(NHC)Au(I)-H的直接亲核氢化物攻击引发的,随后[(NHC)Au(I)]+部分在炔的面外键的帮助下迁移到对角线对面。然而,在(NHC)Au(I)-F的加入中,[(NHC)Au(I)]+部分作为路易斯酸起作用,首先激活炔烃,然后是氟化物的攻击。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding the reaction mechanism of anti-addition of (NHC)Au(i)–H and (NHC)Au(i)–F across alkyne†
The experimentally observed anti-addition reactions of (NHC)Au(i)–H with dimethyl acetylenedicarboxylate (DMAD) MeOOCCCCOOMe and (NHC)Au(i)–F with phenylacetylene MeCCPh are intriguing and deserve more in-depth study. In this work, with the aid of density functional theory (DFT) calculations and intrinsic bond orbital (IBO) analysis, we systematically investigated the addition reactions of (NHC)Au(i)–X (X = H, Me and halides) with different alkynes. We found that the nature of the two anti-addition reactions is different. The addition of (NHC)Au(i)–H is initiated by a direct nucleophilic hydride attack from (NHC)Au(i)–H, followed by migration of the [(NHC)Au(i)]+ moiety to the diagonally opposite side with the aid of the out-of-plane π-bond of the alkyne. However, in the addition of (NHC)Au(i)–F, the [(NHC)Au(i)]+ moiety functions as a Lewis acid to initially activate the alkyne, followed by the fluoride attack.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


