钯(II)催化氧三环烯烃与烷基苯胺的对映选择性去对称开环:对映富集功能化环己烯的有效途径
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-28
DOI:10.1039/d5qo00118h
引用次数: 0
摘要
我们在此公开了一种实用的杂环烯烃与烷基苯胺的对映选择性去对称开环偶联,该偶联由Pd(II)催化的氨基化级联实现,可以高效地获得具有四个连续密集功能化碳立体中心的多种吲哚环己烯,收率高,具有优异的非对映和对映选择性。代表性的转化后的其他有价值的对映体富集功能化环己烯衍生物证明了这种合成方法的合成潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
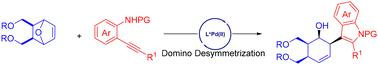
Palladium(ii)-catalyzed enantioselective desymmetrization ring opening of oxabicyclic alkenes with alkynylanilines: efficient access to enantioenriched functionalized cyclohexenes†
We disclose herein a practical enantioselective desymmetrization ring-opening coupling of oxabicyclic alkenes with alkynylanilines, enabled by Pd(ii)-catalyzed aminopalladation cascade, allowing efficient access to a diverse set of indolated cyclohexenes bearing four contiguous densely functionalized carbon stereocenters in high yields with excellent diastereo- and enantioselectivities. Representative post-transformations to other valuable enantioenriched functionalized cyclohexene derivatives demonstrate the synthetic potential of this methodology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


