福霉素A和吡唑脲生物合成途径的修正揭示了吡唑核心形成过程中d-谷氨酸和一个隐性n -酰化步骤的特异性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
Formycin A和pyrazofurin是两种天然存在的吡唑衍生的c -核苷,具有抗菌和抗病毒活性。虽然早期的研究已经确定了c -糖苷键形成的化学过程以及甲霉素A和吡唑呋喃生物合成的后续步骤,但吡唑环本身是如何构建的仍然是难以捉摸的。虽然先前报道吡唑环上N-N键的形成涉及由肼合成酶PyfG催化的n6 -羟化赖氨酸和l-谷氨酸的偶联,但本文显示PyfG及其同源物ForJ识别d-谷氨酸而不是l-谷氨酸。ForJ/PyfG催化的肼产物经nadd依赖性氧化还原酶ForL处理后释放α-肼基d-谷氨酸。此外,α-肼基d-谷氨酸与atp把握连接酶ForM/PyfJ催化的氨基酸的n -酰化是fad依赖的氧化还原酶r /PyfK识别c - α- n键脱氢从而形成腙中间体所必需的。这项工作不仅证明了d-谷氨酸是肼生物合成的正确底物,而且揭示了吡唑核心组装中一个隐式的n -酰化步骤。因此,这些结果为在天然产物中很少见到的吡唑环的生物合成提供了重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
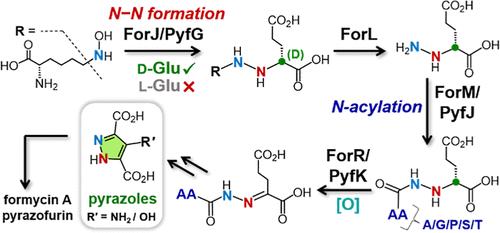
Revision of the Formycin A and Pyrazofurin Biosynthetic Pathways Reveals Specificity for d-Glutamic Acid and a Cryptic N-Acylation Step During Pyrazole Core Formation
Formycin A and pyrazofurin are two naturally occurring pyrazole-derived C-nucleosides with antibacterial and antiviral activities. While earlier studies have established the chemistry of C-glycosidic bond formation as well as the subsequent steps in the biosynthesis of formycin A and pyrazofurin, how the pyrazole ring itself is constructed remains elusive. While N–N bond formation in the pyrazole ring was previously reported to involve coupling of N6-hydroxylated l-lysine and l-glutamic acid catalyzed by the hydrazine synthetase PyfG, herein PyfG and its homologue ForJ are shown instead to recognize d-glutamate instead of l-glutamate. The hydrazine product of ForJ/PyfG catalysis then releases α-hydrazino d-glutamic acid upon processing by the NAD-dependent oxidoreductase ForL. Furthermore, N-acylation of α-hydrazino d-glutamate with an amino acid catalyzed by the ATP-grasp ligase ForM/PyfJ is indispensable for recognition by the FAD-dependent oxidoreductase ForR/PyfK to perform dehydrogenation of the Cα–N bond and thereby form a hydrazone intermediate. This work not only demonstrates that d-glutamic acid is the correct substrate for hydrazine biosynthesis but also reveals a cryptic N-acylation step in the assembly of the pyrazole core. These results thus provide significant insights into the biosynthesis of pyrazole rings that are rarely seen in natural products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


