磷(V)的催化对映选择性亲核脱对称:一种模块化制备磷酸酯的三相策略
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
具有至少一个P-N键而没有P-C键的手性磷酸盐在核苷磷酸酯前药中具有重要的应用价值。尽管有选择性地构建各种手性有机磷实体的方法,但仅由杂原子取代的p -立体中心通常依赖于非对映体合成。在这里,我们提出了一种催化对映选择性去对称策略,使用亲电性磷试剂与三个离去基作为底物,使各种醇和胺的三相亲核攻击成为可能。通过在磷原子周围产生广泛可能的取代基组合,这种合成策略可能加速合成和筛选具有生物活性的磷酸酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
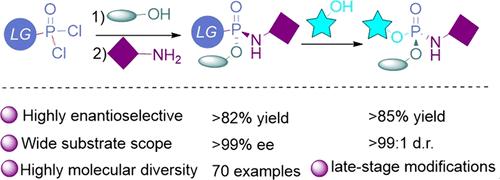
Catalytic Enantioselective Nucleophilic Desymmetrization at Phosphorus(V): A Three-Phase Strategy for Modular Preparation of Phosphoramidates
Chiral phosphoramidates characterized by at least a P–N bond without a P–C bond demonstrate a significant applicative value within nucleoside phosphoramidate prodrugs. Despite the availability of methodologies for the selective construction of diverse chiral organophosphorus entities, achieving P-stereocenters solely substituted by heteroatoms often relies on diastereomeric synthesis. Here, we present a catalytic enantioselective desymmetrization strategy using an electrophilic phosphorus reagent with three leaving groups as a substrate, enabling a three-phase nucleophilic attack with various alcohols and amines. By generating a broad range of possible substituent combinations around phosphorus atoms, this synthetic strategy may expedite the synthesis and screening of biologically active phosphoramidates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


