在一种新型PMM2-CDG小鼠模型中,基于aav的基因替代疗法可以预防和停止异常神经表型的表现。
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
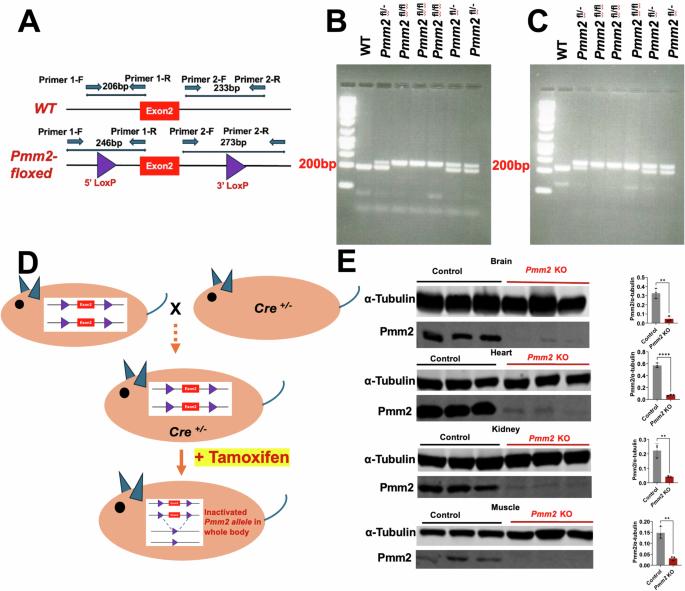
遗传性磷酸腺苷交换酶2 (PMM2)缺乏症,也称为PMM2-CDG,是最常见的n -连锁先天性糖基化(CDG)疾病,在某些人群中发病率约为2万分之一。患者表现出一系列症状,神经系统受累是一个突出特征,通常表现为最初的临床体征,范围从孤立的神经功能缺损到严重的多器官功能障碍。鉴于缺乏治疗方法,两岁以下儿童死亡率高,加上终生发病率高,迫切需要创新的治疗方法。为了解决这一未满足的需求,我们开发了一种他莫昔芬诱导的Pmm2敲除(KO)小鼠模型,该模型具有广泛的Pmm2组织表达缺陷。迄今为止,通过复合表型评分系统和开放场测试,小鼠模型的表征显示了与PMM2-CDG相关的不同神经表型。值得注意的是,通过AAV9-PMM2基因替代疗法增加PMM2可以预防和停止动物中PMM2 KO诱导的疾病相关神经表型。这些发现强调了AAV9-PMM2基因替代治疗PMM2-CDG的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
AAV-based gene replacement therapy prevents and halts manifestation of abnormal neurological phenotypes in a novel mouse model of PMM2-CDG
Inherited Phosphomannomutase 2 (PMM2) deficiency, also known as PMM2-CDG, is the most prevalent N-linked congenital disorder of glycosylation (CDG), occurring in approximately 1 in 20,000 individuals in certain populations. Patients exhibit a spectrum of symptoms, with neurological involvement being a prominent feature, often manifesting as the initial clinical sign, and can range from isolated neurological deficits to severe multi-organ dysfunction. Given the absence of curative treatments and a high mortality rate before the age of two, alongside considerable lifelong morbidity, there is an urgent need for innovative therapeutic approaches. To address this unmet need, we developed a tamoxifen-inducible Pmm2 knockout (KO) mouse model with widespread tissue deficiency of Pmm2 expression. Characterization of the mouse model to-date revealed distinct neurological phenotypes relevant to PMM2-CDG, as assessed by the Composite Phenotype Scoring System and Open Field Test. Notably, PMM2 augmentation through AAV9-PMM2 gene replacement therapy prevented and halted the disease-relevant neurological phenotypes induced by Pmm2 KO in the animals. These findings underscored the promise of AAV9-PMM2 gene replacement in managing PMM2-CDG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


