通过氧化还原中性光催化n -乙烯酰亚胺与α-氨基烷基自由基的反应,方便地获得α-(杂)芳基-α-酮-1,3-二胺
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-28
DOI:10.1039/d5qo00239g
引用次数: 0
摘要
在此,我们报告了一种基于氧化还原中性光催化还原自由基-极性交叉过程的新型酰基迁移方案。采用叔胺或 α-硅胺作为自由基来源,在温和的反应条件下,通过烯酰胺与 α-氨基烷基自由基的反应,可以高效地获得各种功能化 α-(杂)芳基-α-酮基-1,3-二胺。此外,自由基加成/酰基迁移级联过程还可扩展到分子内版本。此外,还展示了酰基迁移产物的合成应用。利用通过单电子转移过程轻松生成 α-氨基烷基自由基和 α-酰亚胺碳离子的优势,这一新方法具有底物范围广和无外源还原剂条件的特点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
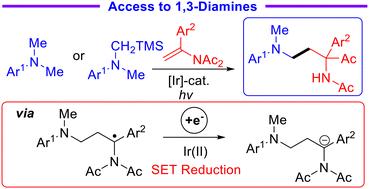
Expedient access to α-(hetero)aryl-α-keto-1,3-diamines via redox-neutral photocatalyzed reactions of N-vinylimides with α-aminoalkyl radicals†
Herein, we report a novel acyl migration protocol based on a reductive radical-polar crossover process enabled by redox-neutral photocatalysis. Employing tertiary amines or α-silylamines as radical sources, modular access to various functionalized α-(hetero)aryl-α-keto-1,3-diamines could be efficiently realized via reactions of enamides with α-aminoalkyl radicals under mild reaction conditions. Additionally, the radical addition/acyl migration cascade process could be extended to the intramolecular version. The synthetic application of acyl migrated products was also demonstrated. Taking advantage of the easy generation of α-aminoalkyl radicals and α-imido carbanions via a single-electron-transfer process, this new procedure features a broad substrate scope and exogenous reductant-free conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


