镍催化的三氟异芳基醛的动力学动力学不对称还原芳基化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这种情况下,我们报道了镍催化的醛与外消旋三氟化杂二芳基的动力学动力学不对称还原芳基化,以高度非映对和对映选择性的方式提供了一系列轴手性杂二芳基,其中中心手性仲醇部分。同时控制产物的轴向和中心立体元素的方法是将构型不稳定的杂二芳基镍配合物在醛的甲酰基上进行立体选择性的亲核加成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
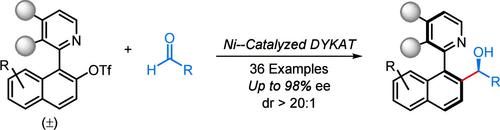
Nickel-Catalyzed Dynamic Kinetic Asymmetric Reductive Arylation of Aldehydes with Heterobiaryl Triflates
In this context, we report a nickel-catalyzed dynamic kinetic asymmetric reductive arylation of aldehydes with racemic heterobiaryl triflates, offering a series of axially chiral heterobiaryls bearing a centrally chiral secondary alcohol moiety in a highly diastereo- and enantioselective manner. The simultaneous control of both axial and central stereogenic elements of the products lies in the stereoselective nucleophilic addition of the configurationally labile hetereobiaryl nickel complex to the formyl group of aldehydes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


