发现毒蕈碱受体M2的关键激活热点
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
M2 muscarinic 受体(M2R)是一种典型的 G 蛋白偶联受体(GPCR),是了解配体识别和 GPCR 激活的模型系统。在这里,我们利用振动光谱学确定了 M2R 被其原生激动剂乙酰胆碱激活的机制。结合诱变、计算化学和有机合成化学,我们的分析发现,乙酰胆碱与构成配体结合位点的氨基酸之一 Asn404 之间的精确距离对于 M2R 的活化非常重要,N404Q 突变体会发生部分类似于活性状态的构象变化。我们发现,连接乙酰胆碱和 Asn404 的水分子形成了一个精确而灵活的氢键网络,引发了 M2R 跨膜螺旋 6 的向外运动。与这一观察结果一致的是,通过在乙酰胆碱的 α 位或β位进行化学修饰来破坏这一氢键网络并不能激活 M2R。总之,我们的研究结果指出 Asn404 是一个关键残基,它既能感知乙酰胆碱的结合,又能诱导 M2R 激活。本文章由计算机程序翻译,如有差异,请以英文原文为准。
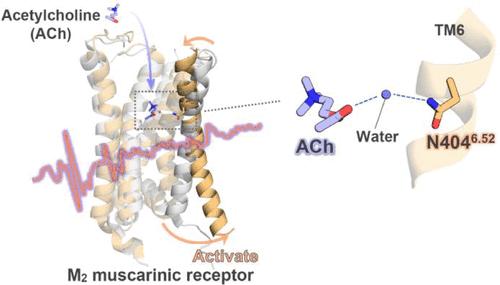
Discovering Key Activation Hotspots in the M2 Muscarinic Receptor
The M2 muscarinic receptor (M2R) is a prototypical G protein-coupled receptor (GPCR) that serves as a model system for understanding ligand recognition and GPCR activation. Here, using vibrational spectroscopy, we identify the mechanisms governing M2R activation by its native agonist, acetylcholine. Combined with mutagenesis, computational chemistry, and organic synthetic chemistry, our analyses found that the precise distance between acetylcholine and Asn404, one of the amino acids constituting the ligand-binding site, is important for M2R activation and that the N404Q mutant undergoes partial active state-like conformational changes. We discovered that a water molecule bridging acetylcholine and Asn404 forms a precise and flexible hydrogen bond network, triggering the outward movement of transmembrane helix 6 in M2R. Consistent with this observation, disruptions in this hydrogen bond network via chemical modification at the α- or β-position of acetylcholine failed to activate M2R. Collectively, our findings pinpoint Asn404 as a critical residue that both senses acetylcholine binding and induces M2R activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


