未来糖蛋白药物的概念:巯基连接α- n -乙酰半乳糖胺环肽作为微型巨噬细胞激活因子模型的合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
糖蛋白通常被认为是候选药物。然而,翻译后聚糖附着的调控仍然是一个问题。我们假设用硫原子取代糖苷键中的氧原子可以稳定不稳定的糖苷键,从而改善药代动力学。在本研究中,我们重点研究了携带O-linked n - acetyl半乳糖胺(GalNAc)的巨噬细胞激活因子(MAF),并创建了与MAF相关的微型糖肽。设计了MAF的部分结构,在三个氨基酸残基上发生化学突变,其中苏氨酸被半胱氨酸(Cys)取代,导致巯基糖苷连接的GalNAc和由于二硫键而形成构象稳定的环肽。采用GalNAc-Cys固相合成多肽,合成了所需的环状糖肽。在GalNAc-Cys的合成中,根据软硬酸碱的概念进行糖基化反应,成功地利用三氯乙酸糖基和氟化物与Cys中的巯基偶联。GalNAc-Cys也被评价为α- galnac -酶的底物,并被证明抵抗水解,支持我们的概念。合成的环状微型MAF诱导lps辅助IL-12产生,并对α- galnac -酶具有抗性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
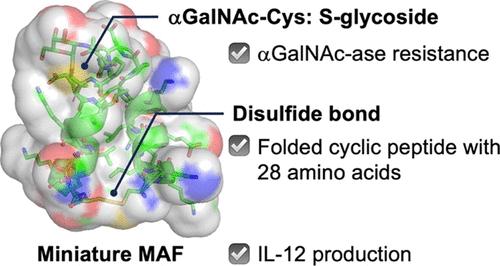
Concept of Future Glycoprotein Drugs: Synthesis of a Thioglycosidically Linked α-N-Acetylgalactosamine-Carrying Cyclic Peptide as a Model of Miniature Macrophage Activating Factor
Glycoproteins are often considered as drug candidates. However, the regulation of post-translational glycan attachment remains an issue. We hypothesized that replacing the oxygen atom in the glycosidic linkage with sulfur atoms would stabilize the labile linkage against glycosidases, resulting in improved pharmacokinetics. In this study, we focused on the macrophage-activating factor (MAF) carrying O-linked N-acetylgalactosamine (GalNAc) and creating a miniature glycopeptide associated with MAF. A partial structure of MAF with a chemical mutation at three amino acid residues was designed in which threonine was replaced with cysteine (Cys), leading to a thioglycosidically linked GalNAc and a conformationally stable cyclic peptide due to the disulfide bond. GalNAc-Cys was used in solid-phase peptide synthesis, and the desired cyclic glycopeptide was synthesized. In the synthesis of GalNAc-Cys, glycosylation reactions were carried out based on the hard and soft acids and bases concept, where glycosyl trichloroacetimidate and fluoride were successfully used to couple with the thiol group in Cys. GalNAc-Cys was also evaluated as a substrate of α-GalNAc-ase and was shown to resist hydrolysis, supporting our concept. The synthesized cyclic miniature MAF induced LPS-assisted IL-12 production and resisted against α-GalNAc-ase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


