以假糖基立体定向合成1,2,3-三取代环丙烷
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
构造具有三个连续立体中心的光学纯1,2,3-三取代环丙烷的策略设计是合成有机化学中的一个艰巨挑战。在本文中,我们报道了一种简单且高度对映特异性的假糖基转化为手性1,2,3-三取代环丙烷,具有三个连续的立体中心,涉及立体特异性[1,4]-Wittig重排。研究了官能团取向和构象偏好的影响。成功的克级制备和随后的衍生化反应产生了多种具有多个立体中心的环丙烷支架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
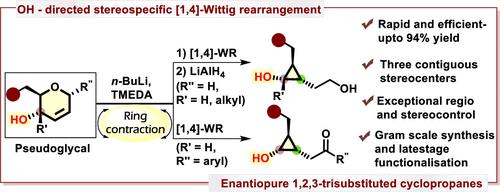
Stereospecific Synthesis of 1,2,3-Trisubstituted Cyclopropanes from Pseudoglycals
Strategic design for constructing optically pure 1,2,3-trisubstituted cyclopropanes with three contiguous stereocenters represents a formidable challenge in synthetic organic chemistry. Herein, we report a simple and highly enantiospecific transformation of pseudoglycals into chiral 1,2,3-trisubstituted cyclopropanes, featuring three consecutive stereocenters, involving a stereospecific [1,4]-Wittig rearrangement. The effects of functional group orientation and conformational preferences were studied. Successful gram-scale preparations and subsequent derivatization reactions yielded various cyclopropane scaffolds with multiple stereocenters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


