在新加坡门诊实施预防性药物基因组学检测的可行性研究(IMPT研究)。
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
鉴于初级保健机构中预防性药物基因组学(PGx)检测的相关数据有限,我们设计了一项研究,以评估在门诊诊所实施预防性PGx服务的可行性,旨在评估在初级保健中实施预防性PGx检测的实用性和挑战,及其对临床工作流程和患者护理的影响。这项前瞻性研究于2022年10月至2023年8月在新加坡的五家门诊诊所进行。研究招募年龄在21 - 65岁之间,有目标慢性疾病病史或风险的患者,或接受29种pgx相关药物之一的患者。患者的口腔样本使用基于多基因qpcr的5种药物基因的21个等位基因变异体进行处理。研究人员对研究参与者和临床医生进行了调查,以评估他们对PGx检测的看法和结果。在222名患者中,95%的患者至少有一种临床可操作的变异。在这些患者中,113例报告至少服用了29种研究药物中的一种,其中21.2%的患者根据其PGx结果接受了至少一种临床可操作的建议。150例患者(67.6%)参加了测试后随访调查。其中,70%的人在收到检查报告后感到轻松和快乐,并表示服用处方药的信心增加了。此外,临床医生认为有必要对PGx检测和保险范围制定更明确的法律法规,以加强PGx检测的未来采用。鉴于几乎所有检测患者中临床可操作变异的高患病率,本研究强调了在新加坡初级保健诊所进行预防性PGx检测的可行性和临床益处。临床试验注册:本研究在ClinicalTrials.gov注册,编号NCT05504135,注册日期为2022年8月17日。本文章由计算机程序翻译,如有差异,请以英文原文为准。
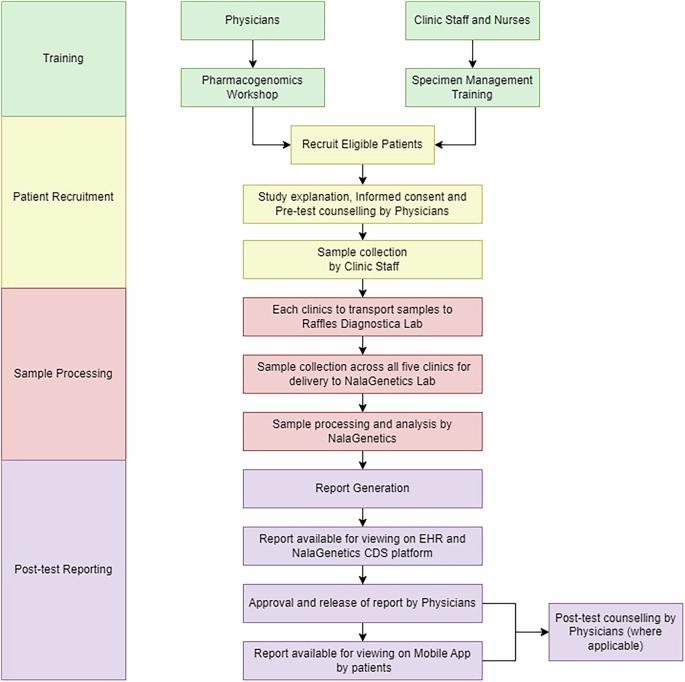
A feasibility study on implementing pre-emptive pharmacogenomics testing in outpatient clinics in Singapore (IMPT study)
In view of the limited data related to preemptive pharmacogenomics (PGx) testing in the primary care setting, we designed a study to assess the feasibility of implementing preemptive PGx services at outpatient clinics, with the aim to assess the practicality and challenges of implementing preemptive PGx testing within primary care, and its impact on clinical workflows and patient care. This prospective study was conducted between October 2022 and August 2023 at five outpatient clinics located in Singapore. Patients aged 21 to 65 with a reported history or risk of developing any of the target chronic conditions or any patients receiving one of the 29 PGx-associated medications were recruited. Patients’ buccal samples were processed using a multi-gene qPCR-based panel of 21 allele variants of five pharmacogenes. Surveys were administered to study participants and clinicians to assess their perceptions and outcomes related to PGx testing. Among the 222 patients, 95% had at least one clinically actionable variant. Of these patients, 113 reported taking at least one of the 29 studied drugs, with 21.2% of them receiving at least one clinically actionable recommendation based on their PGx results. A total of 150 patients (67.6%) participated in the post-test follow-up survey. Among them, 70% expressed feeling relieved and happy upon receiving their test reports and reported increased confidence in taking their prescribed medication. Furthermore, clinicians identified the necessity for clearer legal regulations regarding PGx testing and insurance coverage to enhance future adoption of PGx testing. Given a high prevalence of clinically actionable variants in almost all tested patients, this study underscores the feasibility and clinical benefits of preemptive PGx testing in primary care clinics in Singapore. Clinical Trial Registration: This study is registered with ClinicalTrials.gov, identifier NCT05504135, with the registration date of August 17, 2022.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


