纳米Chevrel相Mo6S8中Li+的原位嵌入对硝基芳烃的高效电化学还原
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电化学硝基arene还原可以通过控制电位和电流来操纵多个电子和质子的转移,从而在环境条件下实现苯胺的绿色生产,但在使用非贵重催化剂的ph中性电解中仍然存在挑战。本文首次提出了具有高导电性和可插入框架的Chevrel相Mo6S8,作为具有突出性能的低成本候选物,更重要的是,作为揭示硝基芳烃电还原阳离子效应的新平台。在0.1 M LiClO4中,在−0.45 V (vs RHE)条件下,由聚合物限制硫化得到的纳米Mo6S8在将4-硝基苯乙烯还原为4-氨基苯乙烯方面具有很高的产率(~ 95%)和法拉第效率(~ 99%),优于一系列金属硫化物甚至贵金属。结合实验和理论分析,确定了插层相关的阳离子效应,扩展了目前仅限于电极外亥姆霍兹平面的知识。在电解过程中原位插入Li+改善了Mo6S8的电子构型,从而通过质子耦合电子转移机制促进硝基在低配位Mo位点上的吸附进行氢化。此外,从广泛的底物中高效地电合成具有保守还原基的苯胺衍生物,突出了Mo6S8在电化学精炼方面的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
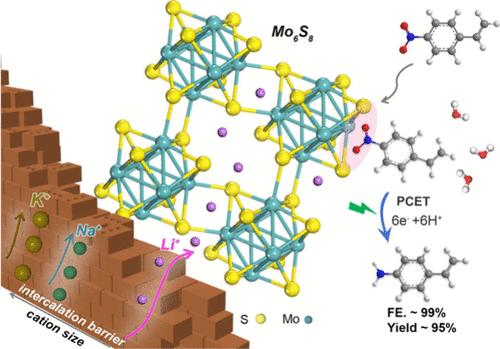
In Situ Li+ Intercalation into Nanosized Chevrel Phase Mo6S8 toward Efficient Electrochemical Nitroarene Reduction
Electrochemical nitroarene reduction enables the green production of anilines at ambient conditions thanks to the manipulated transfer of multiple electrons and protons via controlling potentials and currents, but challenges remain in pH-neutral electrolysis using nonprecious catalysts. Here, Chevrel phase Mo6S8 with high conductivity and insertable frameworks is proposed for the first time as a cost-efficient candidate with prominent performance and, more importantly, as a new platform to unravel cation effects on nitroarene electroreduction. Nanosized Mo6S8 derived from polymer-confined sulfidation affords a high yield (∼95%) and Faradaic efficiency (∼99%) for reducing 4-nitrostyrene to 4-aminostyrene at −0.45 V (vs RHE) in 0.1 M LiClO4, outperforming a series of counterparts of metal sulfides and even noble metals. The combination of experimental and theoretical analyses identifies an intercalation-correlated cation effect, expanding the current knowledge limited to the outer Helmholtz plane of electrodes. In situ Li+ intercalation into Mo6S8 cavities during electrolysis ameliorates the electronic configurations and thereby promotes the adsorption of the nitro group on low-coordinated Mo sites for hydrogenation via a proton-coupled electron transfer mechanism. Furthermore, the efficient electrosynthesis of aniline derivatives with conserved reducing groups from a wide range of substrates highlights the promise of Mo6S8 for electrochemical refinery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


