双abolene合成酶的表征和工程揭示了一个不寻常的氢化物转移和对单、双、三环倍半萜形成至关重要的关键残基
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
倍半萜合成酶(STSs)催化碳阳离子级联反应,具有不同的氢移位和环化模式,从而产生结构多样的倍半萜骨架。然而,决定STS产物分布的氢位移和环化的分子基础仍然是谜。本研究在sydonol的生物合成中鉴定了一个难以捉摸的STS SydA,该SydA合成了一个具有独特的饱和末端垂链异戊烷的新型双abolene型倍半萜6。来自同位素标记实验、SydA及其变体的晶体结构、量子化学计算和诱变实验的大量证据揭示了6的形成机制,包括不寻常的1,7-氢化物移位,这可能是单环、双环和三环产物的关键分支点。基于结构的工程导致SydA变体促进不同的反应途径,导致产生双环α-cuprenene和(+)-β- chamigene和三环7-epi-β-cedrene和β-microbiotene。这些发现不仅揭示了一种新的双abolene及其生物合成,而且为氢化物移位和环化的分子基础提供了见解,为工程sts生产复杂的萜类产品铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
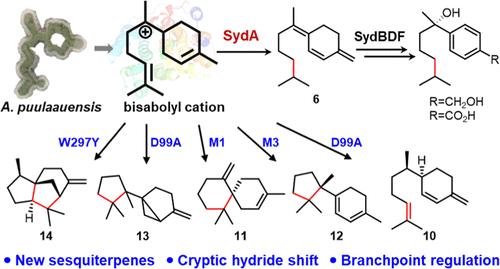
Characterization and Engineering of a Bisabolene Synthase Reveal an Unusual Hydride Shift and Key Residues Critical for Mono-, Bi-, and Tricyclic Sesquiterpenes Formation
Sesquiterpene synthases (STSs) catalyze carbocation cascade reactions with various hydrogen shifts and cyclization patterns that generate structurally diverse sesquiterpene skeletons. However, the molecular basis for hydrogen shifts and cyclizations, which determine STS product distributions, remains enigmatic. In this study, an elusive STS SydA was identified in the biosynthesis of sydonol, which synthesized a new bisabolene-type sesquiterpene 6 with a unique saturated terminal pendant isopentane. Extensive evidence from isotope labeling experiments, crystal structures of SydA and its variant, quantum chemical calculations, and mutagenesis experiments reveal a plausible mechanism for the formation of 6 involving an unusual 1,7-hydride shift, which may be a key branchpoint for monocyclic, bicyclic, and tricyclic products. Structure-based engineering resulted in SydA variants that promote different reaction pathways, leading to the production of bicyclic α-cuprenene and (+)-β-chamigrene and tricyclic 7-epi-β-cedrene and β-microbiotene. These findings not only reveal a new bisabolene and its biosynthesis but also provide insights into the molecular basis of the hydride shifts and cyclizations, which pave the way for engineering STSs to produce complex terpenoid products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


