具有优异体内拉曼成像和光热性能的SICTERS小分子的分子工程
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基于拉曼的治疗在敏感的实时成像和治疗方面显示出巨大的潜力。然而,这些先进的材料,主要依赖于SERS技术,遇到了关于底物生物安全性的临床问题。在此,我们分子工程了一个全新的无底物SICTERS小分子,即BTT-TPA(双噻基取代苯并三唑硒二唑衍生物结构),具有超灵敏的拉曼信号和基于自堆叠的优异光热效应。力学研究证实,BTT保持了SICTERS所需的平面结构和多循环畸变振动。TPA增强了供体-受体相互作用,使BTT的拉曼灵敏度高于先前报道的SICTERS分子;同时作为分子转子,将光热转换效率提高到67.44%,优于大多数现有的sers基光热材料。在小鼠原位结肠癌肿瘤模型中,BTT-TPA NPs在拉曼成像引导下具有良好的光热清除原发和转移性肿瘤的作用,显著降低复发率。本研究提出了无底物SICTERS小分子在体内基于拉曼的治疗应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
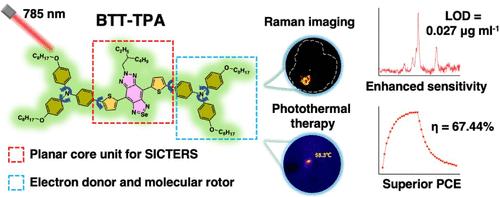
Molecular Engineering of a SICTERS Small Molecule with Superior In Vivo Raman Imaging and Photothermal Performance
Raman-based theranostics has demonstrated great potential for sensitive real-time imaging and treatment. However, these advanced materials, primarily depending on the SERS technique, encounter clinical concerns regarding substrate biosafety. Herein, we molecularly engineered a de novo substrate-free SICTERS small molecule, namely BTT–TPA (bis-thienyl-substituted benzotriazole selenadiazole derivative structures), possessing both ultrasensitive Raman signals and excellent photothermal effects based on self-stacking. The mechanistic studies confirm that BTT maintains the planar structure with polycyclic distorted vibrations required for SICTERS. TPA enhances the donor–acceptor interaction, yielding a Raman sensitivity of BTT higher than previously reported SICTERS molecules; it also acts as a molecular rotor, increasing the photothermal conversion efficiency to 67.44%, which is superior to most of the existing SERS-based photothermal materials. In the tumor model of mouse orthotopic colon cancer, BTT–TPA NPs demonstrate a great Raman imaging-guided photothermal therapy effect in eliminating primary and metastatic tumors, remarkably decreasing the recurrence rate. This work puts forward substrate-free SICTERS small molecules toward Raman-based theranostic applications in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


