环状脂肪沉积肽Anikasin的半自动化全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
实现了源自假单胞菌的环状脂二肽anikasin的全合成。利用去肽结构单元和分支 d-allo-Thr 残基上的平衡保护基团,在合成器上以半自动方式完成了合成。缓冲脱保护将副反应降至最低,并得到了合成的阿尼卡星及其对映体。生物活性研究表明,阿尼卡星的作用模式直接源于其理化性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
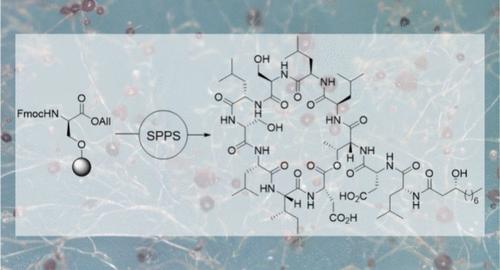
Semiautomated Total Synthesis of the Cyclic Lipodepsipeptide Anikasin
The total synthesis of Pseudomonas-derived cyclic lipodepsipeptide anikasin was achieved. Using a depsipeptide building block and balanced protecting groups on the branching d-allo-Thr residue, the synthesis was established semiautomatically on a synthesizer. Buffered deprotections minimized side reactions and afforded synthetic anikasin and its enantiomer. Biological activity studies indicated that anikasin’s mode of action is directly resulting from its physicochemical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


