宝石双硼光诱导组装不同同丙烯基硼酸盐
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
与在光催化条件下通过四配位硼生成烷基自由基的烷基单硼或1,2-二硼相比,宝石二硼作为底物参与这类反应仍有待开发。在此,我们报告了一种利用宝石二硼作为起始材料生成α-硼基自由基的方法,然后与各种烯烃反应,成功有效地构建了各种高价值的均烯丙基硼酸盐;同时,还可以顺利地获得宝石-二氟均烯丙基骨架。这种转变显示出广泛的底物范围和对官能团的良好耐受性,增强了宝石二硼作为C-C键构建和生产有价值产品的前体的效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
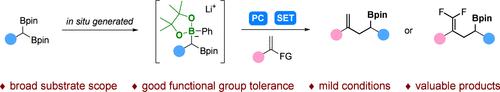
Photoinduced Assembly of Diverse Homoallylic Boronates from gem-Diborons
In comparison to alkyl monoboron or 1,2-diboron, which can generate alkyl radicals via tetracoordinate boron species under photocatalytic conditions, the participation of gem-diborons as substrates in such reactions remains to be developed. Herein, we report a method utilizing gem-diborons as starting materials to generate α-boryl radicals, which then react with various olefins, successfully and efficiently constructing a diverse range of high-value homoallylic boronates; meanwhile, the gem-difluorohomoallylic skeletons could also be smoothly obtained. This transformation demonstrates broad substrate scope and excellent tolerance toward functional groups, enhancing the utility of gem-diboron as precursors for C–C bond construction and the production of valuable products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


