三嗪烷与胺的伴随(3 + 3)环/断裂:多取代二氢嘧啶的电合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了1,3,5-三嗪烷与胺对多取代1,2-二氢嘧啶的电氧化正(3 + 3)环反应。这种不含金属的温和方案具有广泛的官能团耐受性,并且具有未开发的分子支架的杂环以极好的产量构建。从机理上讲,三嗪烷的电氧化和烯胺的亲核反应性导致伴随的环裂解过程,从而产生六元杂环产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
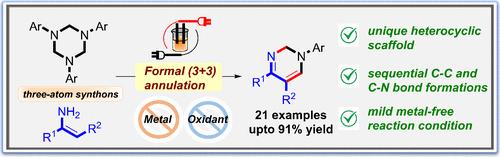
Concomitant (3 + 3) Annulation/Fragmentation of Triazinanes with Enamines: Electrosynthesis of Multisubstituted Dihydropyrimidines
An electro-oxidative formal (3 + 3) annulation of 1,3,5-triazinanes with enamines toward multisubstituted 1,2-dihydropyrimidines is reported. This metal-free mild protocol offers wide functional group tolerance, and heterocycles with an unexplored molecular scaffold were constructed in excellent yields. Mechanistically, the electro-oxidation of triazinane and nucleophilic reactivity of enamine result in a concomitant annulation–fragmentation process, leading to the six-membered heterocyclic product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


