茶树酚内酯倍半萜及其同源物的不对称全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从已知的 (5S)-5,6-二甲基-2-环己烯酮作为手性起始原料开始,通过五到八个步骤的最长线性路线,首次简明地实现了不对称、无保护基团的勃瑞木内酯倍半萜类化合物--木脂勃瑞木内酯 (1)、2α,3α-环氧勃瑞木内酯 A (2) 和 2,3-seco-2,3-olide-1-deoxygen勃瑞木内酯 F (3)--的全合成。这种合成方法的主要特点是对高度官能化的手性醛前体进行 oxa-Pauson-Khand 反应,以一步法的方式形成γ-丁烯内酯融合的三环核心框架。这项研究为环己烷内酯倍半萜的发散合成提供了一个新的战略视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
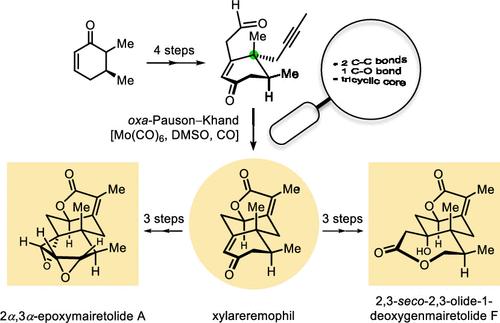
Asymmetric Total Synthesis of Eremophilanolide Sesquiterpene Xylareremophil and Its Congeners
The first asymmetric, protecting group free total synthesis of eremophilanolide sesquiterpenes, xylareremophil (1), 2α,3α-epoxymairetolide A (2), and 2,3-seco-2,3-olide-1-deoxygenmairetolide F (3), is concisely achieved with a longest linear route of five to eight steps, starting from the known (5S)-5,6-dimethyl-2-cyclohexenone as the chiral starting material. This synthetic approach mainly features an oxa-Pauson–Khand reaction of the highly functionalized chiral aldehyde precursor, forging a γ-butenolide-fused tricyclic core framework of eremophilanolides in a one-step manner. This study provides a novel strategic perspective for the divergent synthesis of the eremophilanolide sesquiterpenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


