芳基柄辅助下钯催化的非张力酮C(O) -C键裂解:二芳基酮的研究
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
C-C键裂解反应在分子解构和骨架编辑方面取得了显著进展。在这项研究中,我们描述了钯催化合成二芳基酮通过一系列α-芳基化和有氧氧化C-C键裂解。这种转化具有良好的官能团相容性,特别是对高活性基团,包括- OH, NH2和- CHO。此外,这种方法允许以低催化剂负载方式进行克级合成。它还简化了各种药物或其中间体的合成。机理研究揭示了利用大体积配体和空气作为反应气氛的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
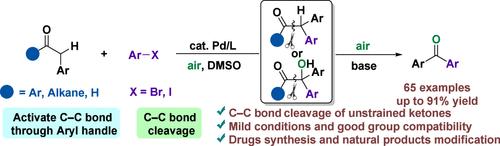
Palladium-Catalyzed C(O)–C Bond Cleavage of Unstrained Ketones Assisted with Aryl Handles: An Approach to Diaryl Ketones
C–C bond cleavage reactions have achieved remarkable progress in molecular deconstruction and skeleton editing. In this study, we describe a palladium-catalyzed synthesis of diaryl ketones through a sequence of α-arylation and aerobic oxidative C–C bond cleavage. This transformation features good functional group compatibility, especially for highly reactive groups, including −OH, NH2, and −CHO. Furthermore, this approach allows for gram-scale synthesis in a low catalyst loading manner. It also streamlines the synthesis of a variety of drugs or their intermediates. Mechanism studies reveal the essential role of utilizing a bulky ligand and the existence of air as the reaction atmosphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


