从丙烯酸酯到具有大跨度可调性和温和条件下化学循环的可持续聚酯平台的模块化访问
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
用传统乙烯基单体制造聚酯是开发可持续聚合材料的最经济的方法之一。对于极性乙烯基来说,虽然人们已经对其转化为内酯的过程进行了广泛的研究,但目前还没有合成聚酯的进一步途径,这可能是由于所获得的内酯具有非受限和不可聚合的性质。在此,我们首次报告了源自最重要的极性乙烯基-丙烯酸酯类的聚酯的简易合成。具体来说,我们从丙烯酸甲酯和含有不同官能团的丙二酸酯以及甲醛中合理地设计和合成了一系列模块化六元内酯。这些单体经过开环聚合反应(ROP)产生了首批丙烯酸酯衍生聚酯,进一步构成了一个独特的聚合物平台,具有广泛的潜在功能和性能,并且在温和的条件下易于化学循环。值得注意的是,所获得的聚酯是侧酯基上具有可调官能团的罕见实例,其对某些材料特性(如玻璃化温度)的影响与聚丙烯酸酯相似,这意味着聚酯和聚丙烯酸酯之间存在潜在的替代关系。此外,由于聚酯首次出现了源自单体γ位的特殊基端二元结构,因此其热性能和回收性能也得到了前所未有的增强:不同的基端二元结构为从完全无定形材料到高结晶材料的大跨度调制提供了独特的途径,高结晶度聚合物的熔融温度与已报道的单取代对应物相比大幅提高了 84 ℃。同时,与其他六元内酯合成的聚酯(其化学循环需要苛刻的条件(150 °C和高真空))相比,这项工作中的宝石二取代聚酯可在更温和的条件下(80 °C和环境压力)完全化学循环成单体。这项研究为今后设计源自极性乙烯基的高性能聚酯提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
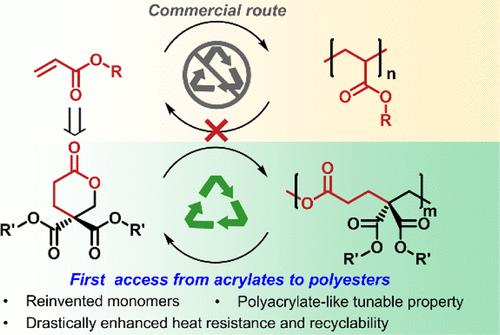
Modular Access from Acrylate to a Sustainable Polyester Platform with Large-Span Tunability and Chemical Circularity under Mild Conditions
Making polyesters with conventional vinyl monomers is one of the most economical ways to develop sustainable polymeric materials. For polar vinyls, while their transformation into lactones has been studied extensively, there exists no further access to synthesizing polyesters, presumably due to the nonstrained and nonpolymerizable nature of the obtained lactones. Herein, we report the first facile synthesis of polyesters that originated from one of the most critical classes of polar vinyls-acrylates. Specifically, a series of modular six-membered lactones were rationally designed and synthesized from methyl acrylate together with malonic esters containing diverse functional groups and formaldehyde. The monomers underwent ring-opening polymerization (ROP) to yield the first acrylate-derived polyesters, which further constitute a unique polymer platform with a large scope of potential functionalities and performances as well as easy chemical circularity under mild conditions. Notably, the obtained polyesters are a rare example featuring tunable functionalities on the side ester groups whose impact on certain material properties (e.g., glass transition temperature) is similar to that of polyacrylates, implying potential replacement between polyesters and polyacrylates. In addition, by presenting the special geminal disubstitutions originally from the monomers’ γ-position for the first time, polyesters also exhibited unprecedentedly enhanced thermal and recycling properties: Variation of the geminal disubstitutions offers a unique access to large-span modulation from completely amorphous to high-level crystalline materials, and the melting temperature of the polymer with high crystallinity was drastically increased by 84 °C compared with the reported monosubstituted counterpart. At the same time, compared with polyesters synthesized from other six-membered lactones whose chemical recycling required harsh conditions (>150 °C and high vacuum), the gem-disubstituted polyesters in this work can undergo complete chemical recycling to monomers under much milder conditions (80 °C and ambient pressure). This study informs the design of future high-performance polyesters derived from polar vinyls.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


