丙炔自由基的低优势共振构型导致多环芳烃保持环五环的生长机制
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
了解多环芳烃(PAHs)的生长对燃烧、天体化学和碳基纳米材料合成至关重要。本研究在理论指导下对丙炔(•C3H3)自由基-自由基组合反应进行了实验研究。将•C3H3加入到三个环五融合的多环芳烃自由基中──1-茚基(•1-C9H7)、苊基(•C12H9)和4h -环五[def]菲壬基(•C15H9)──表明,•C3H3的优势丙基-3-基共振构型与这三个自由基之间的反应一致地产生了具有所有六方环的多环芳烃。而•C3H3较弱的烯-1-基共振构型与三个自由基的反应选择性地保留了环五环,形成了新的六方环。采用分子束采样同步加速器光电离质谱联用和气相色谱-质谱联用技术对中间体和异构体产物进行了观察和鉴定。互补结果表明,烯-1-酰基加成途径具有高选择性,并受热力学控制。本文的研究结果是基于实验能力的结合,它们为碗状多环芳烃的选择性形成提供了新的机制和见解,作为富勒烯和纳米管结构的模板。烯-1-酰基途径的高选择性为环戊烷融合多环芳烃的合成提供了合理的策略,在高温燃烧、星周包封和冷分子云等各种环境下均可实现无障碍、便捷的自由基-自由基反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
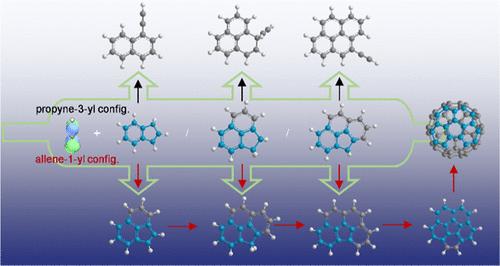
Less-Dominant Resonance Configuration of Propargyl Radical Leads to a Growth Mechanism for Polycyclic Aromatic Hydrocarbons that Preserves the Cyclopenta Ring
Understanding the growth of polycyclic aromatic hydrocarbons (PAHs) is essential for combustion, astrochemistry, and carbon-based nanomaterial synthesis. This study presents theory-guided experiments on radical–radical combination reactions of propargyl (•C3H3). The addition of •C3H3 to three cyclopenta-fused PAH radicals─1-indenyl (•1-C9H7), acenaphthenyl (•C12H9), and 4H-cyclopenta[def]phenanthrenyl (•C15H9)─revealed that the reaction between the dominant propyne-3-yl resonance configuration of •C3H3 and the three radicals consistently produces PAHs with all hexagonal rings, while the reaction between the less dominant allene-1-yl resonance configuration of •C3H3 and the three radicals selectively preserves the cyclopenta ring and forms a new hexagonal ring. Elusive intermediates and isomeric products were observed and identified by combining molecular beam-sampling synchrotron photoionization mass spectrometry with gas chromatography–mass spectrometry. The complementary results suggest a high selectivity of the allene-1-yl addition pathway, which is thermodynamically controlled. The findings presented here are based on a combination of experimental capabilities, and they provide new mechanisms and insights into the selective formation of bowl-shaped PAHs, serving as templates for fullerene and nanotube structures. The high selectivity of the allene-1-yl pathway provides a rational synthetic strategy for cyclopenta-fused PAHs, bearing barrierless and facile radical–radical reaction pathways in various environments, including high-temperature combustion, circumstellar envelopes, and cold molecular clouds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


