分子碘介导的一锅顺序碘环化-脱羧原去碘化:获得密集功能化苯并呋喃
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
描述了一种立体选择性、无金属的密集功能化苯并呋喃的合成方法,通过碱促进的区域选择性碘环化,然后是邻炔芳基2-氰基丙烯酸酯的脱羧原去碘化。醇在碳碳双键上的加成在一次操作中触发了C-O、C-C和C-I键的顺序形成。随后的脱羧和HI的消除提供了最终的苯并fulvene框架,具有外环双键和官能团,如- CN和- OMe,可以很容易地转化为具有生物活性的药物载体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
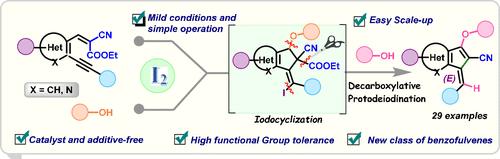
Molecular Iodine-Mediated One-Pot Sequential Iodocyclization–Decarboxylative Protodeiodination: Access to Densely Functionalized Benzofulvenes
A stereoselective, metal-free synthesis of densely functionalized benzofulvenes via base-promoted regioselective iodocyclization followed by decarboxylative protodeiodination of o-alkynylaryl 2-cyanoacrylates is described. Michael addition of alcohol to the carbon–carbon double bond triggers the sequential formation of the C–O, C–C, and C–I bonds in a single operation. Subsequent decarboxylation and the elimination of HI furnish the final benzofulvene framework, featuring an exocyclic double bond and functional groups such as −CN and −OMe that can be easily transformed to afford biologically active pharmacophores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


