转录偶联的AID脱质损伤依赖于elof1相关的RNA聚合酶II
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在适应性免疫中,激活诱导胞苷脱氨酶(AID)将转录偶联损伤(TCD)引入抗体基因,使抗体库多样化。然而,转录和DNA损伤/修复之间的协调仍然难以捉摸。在这里,我们发现转录延伸因子1 (ELOF1)稳定了转录障碍处暂停的RNA聚合酶II (RNAPII),为转录偶联DNA损伤/修复提供了一个平台。通过基因筛选,我们发现ELOF1是AID靶向所必需的,ELOF1缺陷导致小鼠抗体类开关重组缺陷和体细胞超突变。虽然下游转录偶联修复因子对于AID损伤是不可缺少的,但ELOF1通过稳定染色质结合的RNAPII,在机制上促进了TCD和修复。在elof1缺失的细胞中,暂停的RNAPII倾向于与染色质分离,不能招募因子来诱导或修复DNA损伤。我们的研究将ELOF1置于转录偶联DNA代谢过程的中心,并表明RNAPII从延伸到DNA损伤/修复支架的转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
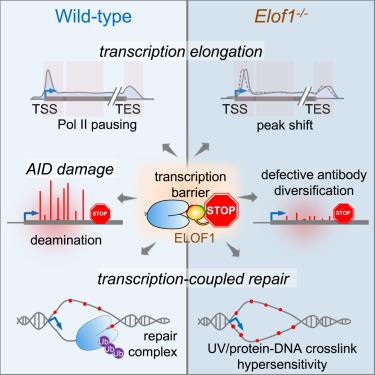
Transcription-coupled AID deamination damage depends on ELOF1-associated RNA polymerase II
In adaptive immunity, transcription-coupled damage (TCD) is introduced into antibody genes by activation-induced cytidine deaminase (AID) to diversify antibody repertoire. However, the coordination between transcription and DNA damage/repair remains elusive. Here, we find that transcription elongation factor 1 (ELOF1) stabilizes paused RNA polymerase II (RNAPII) at transcription barriers, providing a platform for transcription-coupled DNA damage/repair. Using a genetic screen, we discover that ELOF1 is required for AID targeting and that ELOF1 deficiency results in defective antibody class switch recombination and somatic hypermutation in mice. While downstream transcription-coupled repair factors are dispensable for AID damage, ELOF1 mechanistically facilitates both TCD and repair by stabilizing chromatin-bound RNAPII. In ELOF1-deficient cells, paused RNAPII tends to detach from chromatin and fails to recruit factors to induce or repair DNA damage. Our study places ELOF1 at the center of transcription-coupled DNA metabolism processes and suggests a transition of RNAPII from elongation to a DNA damage/repair scaffold.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


