不对称反阴离子定向卤素键催化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
卤素键是一种很有前途的有机催化方法。然而,不对称过程很少,它们的应用仅限于应用手性卤素键供体的少数研究。在此,我们将卤素键与不对称反阴离子定向催化结合起来,提供了这种方法的第一个高度对映选择性的例子。采用手性二磺酰亚胺为反阴离子的强双齿碘(III)基催化剂,对环戊二烯与反式β-硝基苯乙烯的Diels-Alder反应进行了首次不对称有机催化,制备了具有高对映选择性的药物fencamfamine。本文章由计算机程序翻译,如有差异,请以英文原文为准。
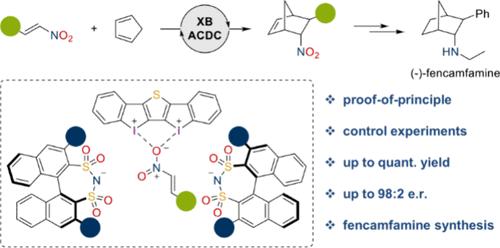
Asymmetric Counteranion-Directed Halogen Bonding Catalysis
Halogen bonding has been established as a promising tool in organocatalysis. Asymmetric processes are nevertheless scarce, and their applications are limited to a few studies applying chiral halogen bond donors. Herein, we combine halogen bonding with asymmetric counteranion-directed catalysis, providing the first highly enantioselective example of such an approach. A strong bidentate iodine(III)-based catalyst with chiral disulfonimides as counteranions is applied in the first asymmetric organocatalysis of the Diels–Alder reaction between cyclopentadiene and trans-β-nitrostyrene, the key step in the synthesis of the drug fencamfamine, which was prepared with high enantioselectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


