协同阴离子-正离子化学使锌金属阳极高度稳定
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
用离子液体(IL)作为锌金属阳极的工程水电解质可以提高锌金属电池(ZMBs)的电化学性能。尽管取得了这些进展,IL的作用及其阴离子和阳离子的机制却很少被研究。在这里,我们介绍了一种新的电解质设计策略,利用卤素基IL协同阴离子-阳离子化学,并阐明了潜在的机制。强且优先吸附的卤素阴离子引导咪唑基阳离子形成贫水双电层(EDL),从而形成富卤素无机界面相。这种协同作用显著减轻了阳极-电解质界面Zn阳极的腐蚀,而富卤化物界面相促进了致密Zn沉积。因此,该电池表现出优异的性能,包括高可逆性(99.74%)和超长循环寿命(20,000次循环)。这种协同的阴离子-正离子化学策略结合了传统的单一固体电解质界面和经典的EDL机制,大大提高了zmb的电化学性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
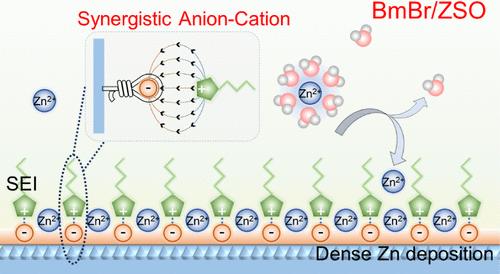
Synergistic Anion–Cation Chemistry Enables Highly Stable Zn Metal Anodes
Engineering aqueous electrolytes with an ionic liquid (IL) for the zinc (Zn) metal anode has been reported to enhance the electrochemical performances of the Zn metal batteries (ZMBs). Despite these advancements, the effects of IL and the mechanisms involving their anions and cations have been scarcely investigated. Here, we introduce a novel electrolyte design strategy that synergizes anion–cation chemistry using a halogen-based IL and elucidates the underlying mechanism. The strongly and preferentially adsorbed halogen anions guide the formation of a water-poor electrical double layer (EDL) by imidazole-based cations, resulting in the formation of a halide-rich inorganic interphase. This synergistic interaction significantly mitigates Zn anode corrosion at the anode–electrolyte interface, while the halide-rich interphase promotes dense Zn deposition. Consequently, the battery exhibits superior performance, including high reversibility (99.74%) and an ultralong cycle life (20,000 cycles). This synergistic anion–cation chemistry strategy combines the traditional single solid electrolyte interphase and the classic EDL mechanism, substantially enhancing the electrochemical performance of ZMBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


