锌水电池共溶剂电解质策略中的动力学补偿机制
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
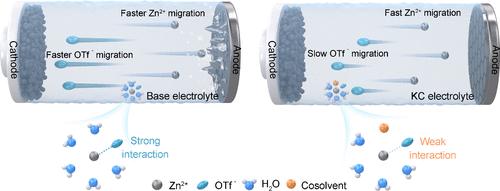
Kinetics Compensation Mechanism in Cosolvent Electrolyte Strategy for Aqueous Zinc Batteries
Aqueous zinc batteries are the ideal choices to realize intrinsically safe energy storage, but parasitic side reactions make it difficult to achieve in practice. Although the cosolvent electrolyte effectively inhibits zinc dendrites and mitigates unexpected side reactions, it brings inevitable kinetics losses. Here, we systematically investigate and compare the interactions between Zn2+ and various oxygen-coordinated cosolvents under pure aqueous environments and the interactions between Zn2+ and OTf– under mixed solvent environments containing different oxygen-coordinated cosolvents. And the differences in the effect of different oxygen-coordinated cosolvents on the solvation structure of Zn2+ and the kinetics of ion migration are quantitatively analyzed and summarized. On this basis, we propose a new kinetics compensation mechanism in cosolvent electrolyte strategy that can compensate the kinetics losses due to the introduction of cosolvents by weakening the anion–cation pair interaction and increasing the Zn2+ transfer number. Theory and experiments both demonstrate that this strategy can achieve kinetics compensation of aqueous zinc batteries while improving the electrochemical performance. This work provides a comprehensive and deep understanding of designing cosolvent electrolytes with superior electrochemical performance. More importantly, the proposed strategy can be applied to other cosolvents with similar properties and other aqueous battery systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


