强耦合Ag/Cu与MXene用于高效串联硝酸还原反应和硝酸锌电池†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
将硝酸盐离子电化学转化为有价值的氨是传统Haber-Bosch工艺的可持续替代方案。然而,硝酸还原电合成氨仍然受到催化活性和法拉第效率低的限制。本工作提出了一种两步串联策略,通过在MXene上集成电荷极化的Ag纳米粒子和Cu纳米团簇来提高电催化性能,从而将硝酸盐还原为氨。与MXene强耦合的Ag纳米颗粒/Cu簇导致Agδ+/Cuδ+极化,分别优先催化NO3−→NO2−和NO2−→NH3转化。合成的Ag/Cu/MXene复合样品的氨收率为10.3 mol gcat。与可逆氢电极相比,在−1.0 V下的法拉第效率为87.7%,并且具有良好的循环稳定性。将该复合材料组装成硝酸锌电池作为阴极;该电池开路电压达到1.81 V,最大输出功率密度为5.75 mW cm−2,具有潜在的应用价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
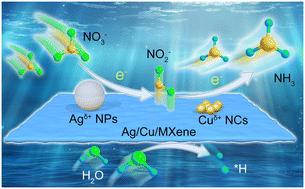
Strongly coupled Ag/Cu with MXene for efficient tandem nitrate reduction reaction and zinc–nitrate batteries†
The electrochemical conversion of nitrate ions into valuable ammonia represents a sustainable alternative to the traditional Haber–Bosch process. However, ammonia electrosynthesis from nitrate reduction is still limited by the low catalytic activity and faradaic efficiency. This work puts forward a two-step tandem strategy for nitrate reduction to ammonia by integrating charge polarized Ag nanoparticles and Cu nanoclusters on MXene to boost the electrocatalytic performance. The strongly coupled Ag nanoparticles/Cu clusters with MXene result in polarized Agδ+/Cuδ+, which preferentially catalyzes NO3− → NO2− and NO2− → NH3 conversions, respectively. The synthesized Ag/Cu/MXene composite sample achieves an ammonia yield rate of 10.3 mol gcat.−1 h−1 and a faradaic efficiency of 87.7% at −1.0 V versus a reversible hydrogen electrode, as well as good cycling stability. The composite was assembled into a zinc–nitrate battery as the cathode; the open-circuit voltage of the battery reaches 1.81 V, with a maximum output power density of 5.75 mW cm−2, demonstrating potential application value.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


