锂硫电池非线性硫溶解度的能量阈值
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
固体溶解度调控机制不明确,难以精确控制其浓度,为加速化学反应提出了挑战。在此,我们使用一个模型系统来确定溶剂类型和比例、浓度和锂盐类型对硫溶解度的影响。我们的发现揭示了硫溶解度与锂盐浓度和复合溶液中不同溶剂的比例之间的非线性关系。重要的是,通过盐阴离子类型调节的溶剂化结构的变化,发现了硫溶解度的显着差异。非线性硫溶解度的定量评价打破了溶质和溶剂分子之间相互作用能的阈值。基于这些发现,可以通过控制盐的浓度和类型来同时调节醚电解质中硫和多硫锂的浓度。因此,硬币电池在有限电解质的情况下,支持了锂硫电池的液-液-固反应途径,并且袋状电池的能量密度为554 Wh kg-1,在所报道的系统中排名最高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
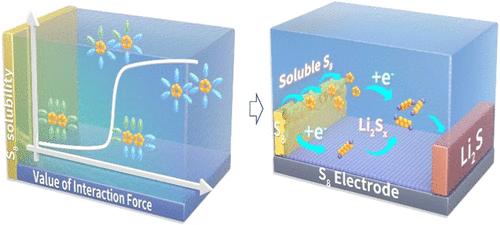
Energy Threshold of Nonlinear Sulfur Solubility for Li–S Batteries
Unclear regulation mechanisms of solid solubility make it difficult to accurately control its concentration, posing challenges for accelerating chemical reactions. Herein, we use a model system to determine the effects of solvent type and ratio, concentration, and type of lithium salt on sulfur solubility. Our findings reveal a nonlinear relationship between sulfur solubility and both lithium salt concentration and the ratio of different solvents in composite solutions. Importantly, significant differences in sulfur solubility are discovered with changes in the solvation structure regulated by the type of salt anion. Quantitative evaluation of nonlinear sulfur solubility is illustrated to be breaking the threshold value of the interaction energy between the solute and solvent molecules. Based on these findings, simultaneous regulation of sulfur and lithium polysulfide concentrations in ether electrolytes can be achieved by controlling the concentration and type of salts. As a result, a liquid–liquid–solid reaction pathway is supported for lithium–sulfur batteries with a finite amount of electrolyte in the coin cell, and the pouch cell shows an energy density of 554 Wh kg–1, ranking at the top level of the reported system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


