IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
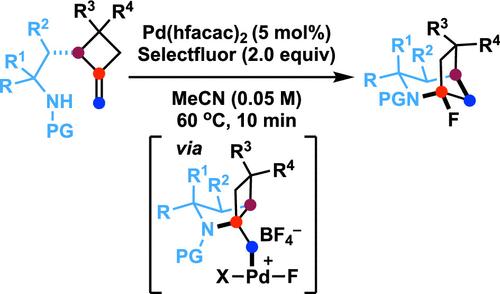
在钯(II)/钯(IV)催化循环下,五-4-烯-1-胺衍生物的环化通常会根据 N 保护基团的不同生成吡咯烷或哌啶。我们在此报告了一种前所未有的钯(II)催化氧化多米诺过程,该过程可将容易获得的 N 保护基 2-(2-氨基乙基)-1-亚甲基环丁烷衍生物转化为 1-氟-2-氮杂双环[3.2.1]辛烷。这种转化在温和的条件下[Pd(hfacac)2 (5.0 mol %)、Selectfluor (2.0 equiv)、MeCN、60 °C, 10 min],通过涉及 5-exo- tridopalladation/Pd(II)-oxidation/chemoselective dyotropic rearrangement/C-F bond-forming reductive elimination 的多米诺序列,构建了三个化学键。值得注意的是,在这些条件下,环化模式与 N 保护基无关。此外,还可以通过明显的反布雷特桥头亚氨基中间体,在双环化合物的桥头位置引入不同的官能团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pd-Catalyzed Strain-Releasing Dyotropic Rearrangement: Ring-Expanding Amidofluorination of Methylenecyclobutanes
Under the Pd(II)/Pd(IV) catalytic cycle, the cyclization of pent-4-en-1-amine derivatives typically yields either pyrrolidines or piperidines depending on the N-protecting group. We report herein an unprecedented Pd(II)-catalyzed oxidative domino process that converts readily accessible N-protected 2-(2-amidoethyl)-1-methylenecyclobutane derivatives to 1-fluoro-2-azabicyclo[3.2.1]octanes. This transformation constructs three chemical bonds under mild conditions [Pd(hfacac)2 (5.0 mol %), Selectfluor (2.0 equiv), MeCN, 60 °C, 10 min] through a domino sequence involving 5-exo-trig amidopalladation/Pd(II)–oxidation/chemoselective dyotropic rearrangement/C–F bond-forming reductive elimination. Notably, the cyclization mode remains independent of the N-protecting group under these conditions. Furthermore, diverse functional groups can be introduced at the bridgehead position of a bicyclic compound via an apparent anti-Bredt bridgehead iminium intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


