IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
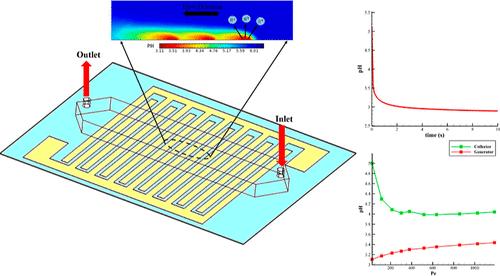
在许多生物和化学应用中,精确控制 pH 值是一个众所周知的必要过程。通过在实验测定中添加缓冲剂(如弱酸和弱碱的盐类)来实现 pH 值的监控。局部 pH 值调整会极大地影响电化学反应的速率和活性。显然,对 pH 值变化的全面研究可以深入了解通过局部控制酸性条件进行电分析的情况。本文采用有限元法对相互咬合的电极阵列进行了全面的实验和数值研究,以研究电极阵列的质量传输,并控制缓冲溶液和非缓冲溶液中的 pH 值变化。此外,还制作了一个微流控装置,利用 pH 指示剂来观察 pH 值的变化,从而进行图像处理和验证数值方法。此外,我们还建立了一个三维数值模型,将电化学系统与流体力学流动结合起来。我们的研究结果表明,在基于扩散的系统中,局部 pH 值高度依赖于电极配置、电流密度和缓冲能力,而初始 pH 值对调节局部 pH 值没有显著影响。通过耦合流体动力学流动,对流传输主导了质子沿流动方向的传输。流体力学直接关系到质子的扩散过程和 pH 值的变化。在这种系统中,可以通过平衡对流效应和修改几何参数(包括微通道高度和电极分离)来加强 pH 值控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comprehensive Study of Transient Mass Transport to Platinum Interdigitated Electrode Arrays to Perform Electrochemical pH Adjustment: Experimental and Numerical
Precise pH control is a well-known and necessary process in many biological and chemical applications. Monitoring pH is achieved by adding buffering agents, such as salts of a weak acid and a weak base, to the experimental assays. Local pH adjustment can significantly affect the rate and activity of the electrochemical reactions. It is evident that a comprehensive study of pH change provides in-depth insight into electroanalysis by locally control acidic conditions. In this article, a finite element method has been employed to perform a comprehensive experimental and numerical study of interdigitated electrode arrays to investigate mass transport to the electrode arrays and control the pH change in the buffered and unbuffered solutions. A microfluidic device has been fabricated to visualize the pH change by using a pH indicator to perform image processing and verify the numerical method. Moreover, a 3D numerical model has been developed to couple an electrochemical system with a hydrodynamic flow. Our results revealed that local pH depends highly on the electrode configuration, current density, and buffering capacity in the diffusion-based system, while initial pH has no significant effect on adjusting local pH. By coupling the hydrodynamic flow, convective transport dominates the proton transport along the flow direction. Flow hydrodynamics directly relates to the diffusion process of the protons and pH variation. In such systems, pH control could be enhanced by balancing convective effects and modifying the geometrical parameters, including microchannel height and electrode separation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


