一石二鸟:铁-蒽分子二元化合物的微秒暗激发态寿命和大笼子逃逸产率
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
铁光敏剂是光化学领域的圣杯,但其激发态寿命短、笼逸率低,限制了铁光敏剂的广泛应用。在这里,通过在铁(III)复合物中添加蒽分子,解决了这两个限制因素,并产生了一种新型二元化合物,其激发态寿命长达 11.5 μs。实现这种超长寿命的关键在于短寿命的铁中心发射激发态的析出,以填充位于类蒽分子上的具有自旋禁止失活的暗三重态。蒽的三重态在乙腈中的效率仅为 10%,但仍能扩大铁基光敏剂的反应范围,现在已包括向 3O2 的能量转移。此外,在一项以甲基紫精为电子受体的原理验证研究中,三重激发态的出现使笼逸产率大幅提高了十倍,从未予取代的复合物的 4.5% 提高到分子二元化合物的 42%。因此,这种新的铁基二元化合物为基于第一排过渡金属的配合物提供了一种补充方法,可替代基于贵金属的成熟类似物。我们相信,进一步的光谱研究和能量接受体的合成修饰以及与光敏剂的连接将是在不久的将来将这些创新的二元化合物用于应用的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
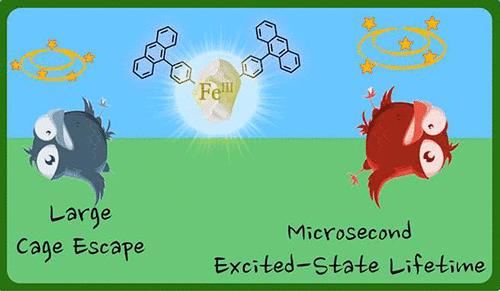
Two Birds, One Stone: Microsecond Dark Excited-State Lifetime and Large Cage Escape Yield Afforded by an Iron–Anthracene Molecular Dyad
Iron photosensitizers represent a holy grail in photochemistry, but their widespread implementation is limited by their short excited-state lifetimes and poor cage escape yields. Here, the introduction of an anthracene moiety appended to an iron(III) complex allowed to solve both limitations and generate a novel dyad exhibiting an extraordinary excited-state lifetime of 11.5 μs. The key to achieving this remarkably long lifetime is the depopulation of the short-lived iron-centered emissive excited state to populate a dark triplet state located on the anthracene-like moiety with a spin-forbidden deactivation. Population of the triplet anthracene only occurs with ∼10% efficiency in acetonitrile but still allows expansion of the scope of reactivity accessible with iron-based photosensitizers, which now encompasses energy transfer to 3O2. In addition, in a proof-of-principle investigation with methyl viologen as electron acceptor, the population of a triplet excited state allows a drastic ten-fold increase of the cage escape yield from 4.5% for the unsubstituted complex to 42% for the molecular dyad. Hence, this new iron-based dyad provides a complementary approach for complexes based on first-row transition metals as alternatives to well-established analogues based on precious metals. We believe that further spectroscopic investigations and synthetic modifications of the energy acceptor and linkage to the photosensitizer will be key to use these innovative dyads for applications in the near future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


