探索半可降解(P^N)配体在氧化还原金催化中的电子和空间效应:在脂肪胺与碘化芳基交叉偶联反应中的应用
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了17种新的(P^N)配体用于氧化还原金的催化,它们在芳基环和氮柄的- C4, - C5和- C6上具有不同的取代基。速率动力学实验表明,芳基环- C4和- C5位置的富电子取代基比贫电子取代基提高了Au(I)与C(sp2) -Br键的氧化加成速率。此外,我们报道了一种前所未有的金催化脂肪胺的芳基化,使用一种富含电子的配体(L6),在- C5位置上有一个- OMe基团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
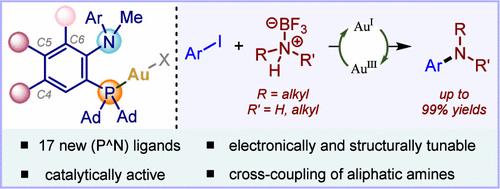
Exploring the Electronic and Steric Effects of Hemilabile (P^N) Ligands in Redox Gold Catalysis: Application to the Cross-Coupling Reaction of Aliphatic Amines with Aryl Iodides
Herein, we report 17 new (P^N) ligands for redox gold catalysis, featuring various substituents at −C4, −C5, and −C6 of the aryl ring and nitrogen handle. Rate kinetics experiments revealed that electron-rich substituents at −C4 and −C5 positions of the aryl ring enhanced the rate of oxidative addition of Au(I) with C(sp2)–Br bonds compared to electron-poor substituents. Further, we report an unprecedented gold-catalyzed arylation of aliphatic amines using an electronically rich ligand (L6) with an −OMe group at the −C5 position.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


