原生18O、33P中SAMHD1的过渡态分析及溶剂动力学同位素效应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
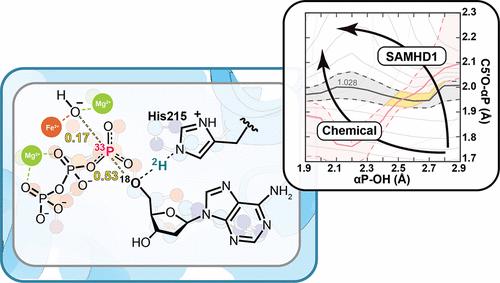
人不育α基序和含hd结构域蛋白1 (SAMHD1)是一种受变构调控的dNTP三磷酸水解酶(dNTP + H2O→dNuc + PPPi),参与脱氧核苷酸调控和DNA修复。我们通过分析SAMHD1在2 ' -脱氧腺苷5 ' -三磷酸(dATP)水解过程中α-磷酸基上的18O和33P一级动力学同位素效应(KIEs)来表征SAMHD1水解过渡态的化学特征。[5′-18O]dATP的内在KIE值为1.028±0.003,[α-33P]dATP的内在KIE值为1.015±0.004,为SAMHD1过渡态的机制细节提供了深入的见解。溶剂2H2O对dATP水解的同位素效应表明,在过渡态转移了一个质子,使溶剂的KIE为3.2±0.1。同位素效应的量子化学匹配支持一个协调的、松散的、高度不对称的DNAN过渡态,进攻的氢氧亲核试剂的鲍林键阶为0.17,离开的脱氧腺苷的鲍林键阶为0.53。从攻击羟基氧到离开5 ' -脱氧腺苷氧的反应配位距离为4.7 Å。溶剂KIE与His215催化位点质子供体向过渡态脱氧腺苷5′-氧的近中点质子转移相一致。这是第一个被表征的三磷酸水解酶过渡态,也是第一次使用33P初级同位素效应来表征磷酸转移酶过渡态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transition State Analysis of SAMHD1 from Primary 18O, 33P, and Solvent Kinetic Isotope Effects
Human sterile alpha motif and HD-domain-containing protein 1 (SAMHD1) is an allosterically regulated dNTP triphosphohydrolase (dNTP + H2O → dNuc + PPPi) involved in deoxynucleotide regulation and DNA repair. We characterized the chemical features of the SAMHD1 transition state for 2′-deoxyadenosine 5′-triphosphate (dATP) hydrolysis by analysis of 18O and 33P primary kinetic isotope effects (KIEs) at the α-phosphoryl of the leaving triphosphate group. The intrinsic KIE values for [5′-18O]dATP of 1.028 ± 0.003 and for [α-33P]dATP of 1.015 ± 0.004 provide insights into the mechanistic details of the SAMHD1 transition state. Solvent 2H2O isotope effects for the hydrolysis of dATP indicate that a single proton is being transferred at the transition state to give a solvent KIE of 3.2 ± 0.1. Quantum chemical matching of the isotope effects supports a concerted, loose, highly asymmetric DNAN transition state with a Pauling bond order of 0.17 to the attacking hydroxide oxygen nucleophile and 0.53 to the departing deoxyadenosine. The reaction coordinate distance is 4.7 Å from attacking the hydroxyl oxygen to departing 5′-deoxyadenosine oxygen. The solvent KIE is consistent with a near-midpoint proton transfer from the His215 catalytic site proton donor to the deoxyadenosine 5′-oxygen in the transition state. This is the first triphosphohydrolase transition state to be characterized and the first use of a 33P primary isotope effect to characterize a phosphotransferase transition state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


