金黄色葡萄球菌毒力因子共价靶向的mRNA展示方法
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
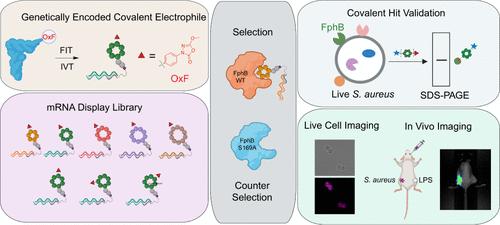
金黄色葡萄球菌(金黄色葡萄球菌)是一种机会性人类病原体,每年在全世界造成100多万人死亡。我们最近发现了一个丝氨酸水解酶家族,称为氟膦酸结合水解酶(Fphs),在宿主的脂质代谢和定植中起重要作用。由于许多这些酶仅在葡萄球菌中表达,因此它们是诊断和治疗的有价值靶点。在这里,我们开发并筛选了高度多样化的环肽文库,使用mRNA显示基因编码的oxadiazolone (Ox)亲电试剂,该试剂先前被证明可以有效和共价抑制多种Fph酶。通过使用WT和催化死FphB进行多轮反选择,我们能够调整最终选择的含有Ox残基的环肽对活性位点丝氨酸的选择性。根据我们的mRNA显示点,我们开发了有效的选择性荧光探针,在活的金黄色葡萄球菌中以个位数纳摩尔浓度标记FphB的活性位点。综上所述,这项工作证明了使用直接遗传编码的亲电试剂来显示共价结合配体的mRNA的潜力,并确定了有效的FphB新探针,这些探针有可能用于诊断和治疗应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An mRNA Display Approach for Covalent Targeting of a Staphylococcus aureus Virulence Factor
Staphylococcus aureus (S. aureus) is an opportunistic human pathogen that causes over one million deaths around the world each year. We recently identified a family of serine hydrolases termed fluorophosphonate binding hydrolases (Fphs) that play important roles in lipid metabolism and colonization of a host. Because many of these enzymes are only expressed in Staphylococcus bacteria, they are valuable targets for diagnostics and therapeutics. Here, we developed and screened highly diverse cyclic peptide libraries using mRNA display with a genetically encoded oxadiazolone (Ox) electrophile that was previously shown to potently and covalently inhibit multiple Fph enzymes. By performing multiple rounds of counter selections with WT and catalytic dead FphB, we were able to tune the selectivity of the resulting selected cyclic peptides containing the Ox residue toward the active site serine. From our mRNA display hits, we developed potent and selective fluorescent probes that label the active site of FphB at single digit nanomolar concentrations in live S. aureus bacteria. Taken together, this work demonstrates the potential of using direct genetically encoded electrophiles for mRNA display of covalent binding ligands and identifies potent new probes for FphB that have the potential to be used for diagnostic and therapeutic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


