达拉单抗/来那度胺/地塞米松治疗不适合移植的新诊断骨髓瘤:MAIA的长期结果
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
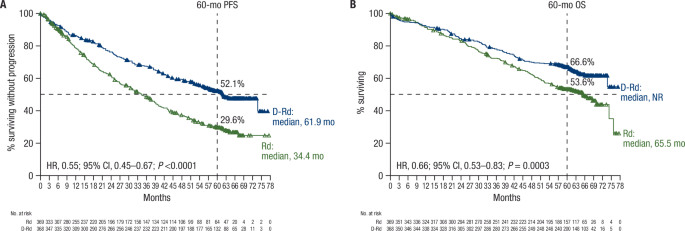
在MAIA研究中,达拉单抗联合来那度胺和地塞米松(D-Rd)在新诊断的多发性骨髓瘤(NDMM)不适合移植的患者中,比单独来那度胺和地塞米松(Rd)改善了无进展生存期(PFS)和总生存期(OS)。我们报告了MAIA的最新疗效和安全性(中位随访,64.5个月),包括按患者年龄(70岁、≥70岁至75岁、≥75岁和≥80岁)进行的亚组分析。总体而言,737名不适合移植的NDMM患者以1:1的比例随机分配到D-Rd或Rd组。D-Rd组与Rd组的主要终点PFS得到改善(中位数为61.9个月vs 34.4个月;风险比[HR], 0.55;95%置信区间[CI], 0.45-0.67;P < 0.0001)。D-Rd组未达到中位总生存期,而Rd组为65.5个月(HR, 0.66;95% ci, 0.53-0.83;p = 0.0003);60个月的OS率分别为66.6%和53.6%。D-Rd获得更高的完全缓解率或更好的完全缓解率(≥CR;51.1% vs 30.1%),最小残留病(MRD)阴性(32.1% vs 11.1%)和持续MRD阴性(≥18个月:16.8% vs 3.3%)与Rd(均P <; 0.0001)相比。D-Rd在各年龄组均表现出临床意义的疗效益处。没有发现新的安全隐患。最新结果(中位随访5年)继续支持在不适合移植的NDMM患者中一线使用D-Rd。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Daratumumab/lenalidomide/dexamethasone in transplant-ineligible newly diagnosed myeloma: MAIA long-term outcomes
In the MAIA study, daratumumab plus lenalidomide and dexamethasone (D-Rd) improved progression-free survival (PFS) and overall survival (OS) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (NDMM). We report updated efficacy and safety from MAIA (median follow-up, 64.5 months), including a subgroup analysis by patient age (<70, ≥70 to <75, ≥75, and ≥80 years). Overall, 737 transplant-ineligible patients with NDMM were randomized 1:1 to D-Rd or Rd. The primary endpoint, PFS, was improved with D-Rd versus Rd (median, 61.9 vs 34.4 months; hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.45–0.67; P < 0.0001). Median OS was not reached in the D-Rd group versus 65.5 months in the Rd group (HR, 0.66; 95% CI, 0.53–0.83; P = 0.0003); estimated 60-month OS rates were 66.6% and 53.6%, respectively. D-Rd achieved higher rates of complete response or better (≥CR; 51.1% vs 30.1%), minimal residual disease (MRD) negativity (32.1% vs 11.1%), and sustained MRD negativity (≥18 months: 16.8% vs 3.3%) versus Rd (all P < 0.0001). D-Rd demonstrated clinically meaningful efficacy benefits across age groups. No new safety concerns were observed. Updated results (median follow-up, >5 years) continue to support frontline use of D-Rd in transplant-ineligible patients with NDMM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


