IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
西方饮食中果糖摄入量较高,但其对抗肿瘤免疫力的影响尚不清楚。果糖在肝脏和小肠中代谢,果糖转运体在肝脏和小肠中高度表达。大多数肿瘤无法利用果糖驱动糖酵解通量,从而使果糖在肿瘤微环境(TME)中富集。如果免疫细胞能表达果糖特异性转运体 GLUT5,就能利用肿瘤微环境中过剩的果糖来增强免疫细胞的效应功能。在这里,我们展示了表达 GLUT5 的 CD8+ T 细胞、巨噬细胞和嵌合抗原受体(CAR)T 细胞在葡萄糖限制的体外条件下都表现出了更好的效应功能。表达 GLUT5 的 T 细胞在体外表现出较高的果糖分解活性,在体内的小鼠合成模型和人类异种移植模型中表现出更高的抗肿瘤功效,尤其是在补充果糖后。总之,我们的数据表明,通过 GLUT5 进行的代谢工程能使免疫细胞有效利用果糖,并在葡萄糖受限的 TME 中增强抗肿瘤免疫力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
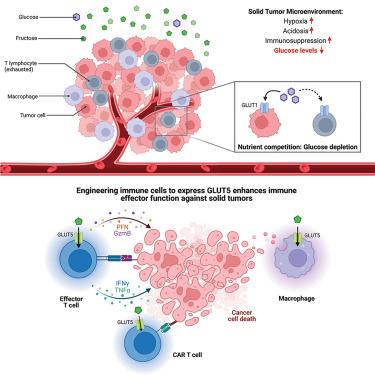
Metabolic engineering to facilitate anti-tumor immunity
Fructose consumption is elevated in western diets, but its impact on anti-tumor immunity is unclear. Fructose is metabolized in the liver and small intestine, where fructose transporters are highly expressed. Most tumors are unable to drive glycolytic flux using fructose, enriching fructose in the tumor microenvironment (TME). Excess fructose in the TME may be utilized by immune cells to enhance effector functions if engineered to express the fructose-specific transporter GLUT5. Here, we show that GLUT5-expressing CD8+ T cells, macrophages, and chimeric antigen receptor (CAR) T cells all demonstrate improved effector functions in glucose-limited conditions in vitro. GLUT5-expressing T cells show high fructolytic activity in vitro and higher anti-tumor efficacy in murine syngeneic and human xenograft models in vivo, especially following fructose supplementation. Together, our data demonstrates that metabolic engineering through GLUT5 enables immune cells to efficiently utilize fructose and boosts anti-tumor immunity in the glucose-limited TME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


