基于1,4-萘醌衍生硫甙的铜催化糖基化方案
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了一种铜催化的糖基化方案,利用2-(对甲氧基苯乙基)-1,4-萘醌-3-硫代糖苷(NQTs)作为有效的糖基供体。这些新的供体以萘醌支架为特征,通过一锅两步合成可以快速有效地制备。此外,它们被成本低廉的Cu(II)盐有效激活,促进与广泛底物的糖基化。NQT供体的实用性进一步证明了它们与潜在活性糖基化策略的兼容性以及它们在多功能一锅合成糖类中的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
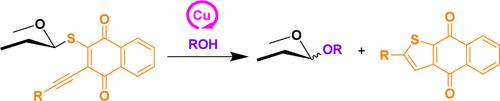
Copper-Catalyzed Glycosylation Protocol Based on 1,4-Naphthoquinone-Derived Thioglycosides
We report a copper-catalyzed glycosylation protocol utilizing 2-(para-methoxyphenylethynyl)-1,4-naphthoquinone-3-thioglycosides (NQTs) as effective glycosyl donors. These novel donors, characterized by a naphthoquinone scaffold, enable rapid and efficient preparation through a one-pot, two-step synthesis. Additionally, they are efficiently activated by cost-effective Cu(II) salts, facilitating glycosylation with a broad range of substrates. The practicality of NQT donors is further demonstrated by their compatibility with latent-active glycosylation strategies and their applicability in the versatile one-pot synthesis of saccharides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


