熔融CaCl2溶剂化电子化学的理论研究
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们利用 ab initio 分子动力学计算研究了熔融 CaCl2 中的钙溶液。溶解的钙原子自发电离并形成溶电子,溶电子的状态随时间而变化,可形成极子(一个溶电子)或双极子(一对溶电子),或处于非定位状态。我们研究了溶质电子与气态 H2、CH4、CO2 和 N2 的反应。溶质电子将 H2 还原成 2H-,将 CH4 还原成 CH3- + H-,将 CO2 还原成 CO22-,将 N2 还原成 2N3-,它们可能成为进一步反应的中间产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
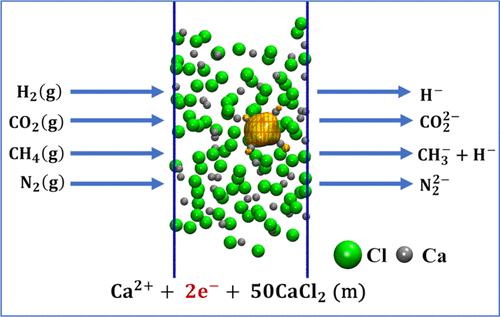
Theoretical Investigation of Solvated Electron Chemistry in Molten CaCl2
We used ab initio molecular dynamics calculations to examine a solution of Ca in molten CaCl2. The dissolved Ca atoms ionize spontaneously and form solvated electrons whose state varies in time to form a polaron (one solvated electron) or a bipolaron (a pair of solvated electrons) or be in a delocalized state. We examined the reaction of solvated electrons with gaseous H2, CH4, CO2, and N2. The solvated electrons reduce H2 to 2H–, CH4 to CH3– + H–, CO2 to , and N2 to 2N3–, which could be intermediates in further reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


