霍克工艺能否用于从聚苯乙烯中生产苯酚?
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚苯乙烯(PS)是一种应用广泛的热塑性聚合物,但其回收率极低,促使人们考虑将废弃PS转化为增值产品的化学转化策略。氧化法已被广泛研究,但它们通常产生苯甲酸,这是一种市场需求相对较低的产品。苯酚是一种体积较大的化学物质,将是一个吸引人的目标,但目前还没有方法将PS转化为苯酚。PS中的重复单元与异丙烯非常相似,异丙烯是通过霍克法生产苯酚的主要原料。在这里,我们研究了将Hock工艺应用于PS的前景,通过苯基C-H键的自氧化生成氢过氧化物,然后通过酸促进氢过氧化物的重排生成苯酚和部分氧化的聚合物。二聚体和三聚体PS模型化合物的实验和计算研究表明,邻近的苯基环施加构象约束,提高了叔苯基C-H键氢原子转移的屏障。这些影响在PS中也很明显,当PS处于Hock工艺条件下时,会导致苯酚的产量降低。这些结果提供了有价值的见解,对寻求适应聚合物原料的小分子反应性的其他努力具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
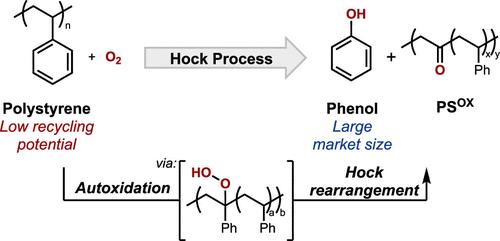
Can the Hock Process Be Used to Produce Phenol from Polystyrene?
Polystyrene (PS) is a widely used thermoplastic polymer, but its very low recycling rate has motivated consideration of chemical conversion strategies to convert waste PS into value-added products. Oxidation methods have been widely studied, but they typically generate benzoic acid, a product with a relatively low market demand. Phenol is a higher volume chemical that would be an appealing target, but no methods currently exist for the conversion of PS into phenol. The repeat unit in PS closely resembles cumene, the primary feedstock used to produce phenol through the Hock process. Here, we investigate prospects for adapting the Hock process to PS, generating hydroperoxides through the autoxidation of benzylic C–H bonds followed by the acid-promoted rearrangement of the hydroperoxides to afford phenol and a partially oxygenated polymer. Experimental and computational studies of dimeric and trimeric PS model compounds show that neighboring phenyl rings impose conformational constraints that raise the barrier to hydrogen-atom transfer from the tertiary benzylic C–H bond. These effects are also evident with PS and contribute to lower yields of phenol when PS is subjected to Hock process conditions. These results provide valuable insights that have important implications for other efforts that seek to adapt small-molecule reactivity to polymeric feedstocks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


