电化学铁钴协同催化C(sp3) -H烯基化
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d5qo00017c
引用次数: 0
摘要
电催化和光催化都成为可持续合成中越来越可行的平台。本研究探索了一种新的光电化学协同策略,利用铁钴双催化体系实现了非定向C(sp3) -H烯基化反应。在光电化学氧化还原催化和铁钴催化的协同作用下,烷烃发生高效的C(sp3) -H烯化反应和脱氢反应,而不需要外部氧化剂,产生氢气作为唯一的副产物。该方法显示了非常规的区域选择性,偏爱在1°C-H键上的烯基化。该研究不仅证明了烯烃作为自由基受体可以影响区域选择性,而且为推进铁催化碳氢功能化提供了一条有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
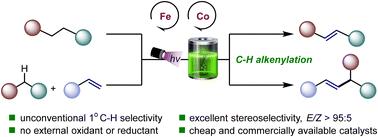
Photoelectrochemical iron–cobalt synergistic catalysis for C(sp3)–H alkenylation†
Electrocatalysis and photocatalysis have both emerged as increasingly feasible platforms in sustainable synthesis. This study explores a novel photoelectrochemical synergistic strategy, achieving non-directed C(sp3)–H alkenylation reaction utilizing an iron–cobalt dual catalytic system. With the synergy of photoelectrochemical redox catalysis and iron–cobalt catalysis, efficient C(sp3)–H alkenylation reaction of alkanes as well as dehydrogenation occur without the need for chemical oxidants or reductants, yielding hydrogen gas as the sole byproduct. The electric current is employed to modulate the oxidation states of the catalysts, fulfilling a role similar to that of external oxidants typically used in transition metal catalysis. This method demonstrates unconventional regioselectivity, with a preference for alkenylation at the 1° C–H bonds. This research not only demonstrates that alkenes as radical acceptors can influence the regioselectivity but also offers a promising pathway for advancing iron-catalyzed C–H functionalization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


