在没有UNG的情况下,SMUG1加工尿嘧啶触发同源重组并选择性地杀死brca1 /2缺陷肿瘤
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对聚(adp -核糖)聚合酶(PARP)抑制剂(PARPis)的耐药性是影响其治疗同源重组(HR)缺陷(HRD)肿瘤有效性的主要障碍。因此,开发HRD肿瘤的替代疗法至关重要。在这里,我们表明靶向尿嘧啶切除途径可以杀死HRD肿瘤,包括那些具有PARPi抗性的肿瘤。我们发现两个主要的尿嘧啶DNA糖基酶UNG和SMUG1之间的相互作用是由核烟酰胺腺嘌呤二核苷酸(NAD+)调节的,它维持UNG在复制叉(RFs)上并抑制SMUG1染色质结合。在没有UNG的情况下,SMUG1在染色质上的保留导致持久的基本位点,APE1切割导致PARP1过度激活,RFs停滞和RAD51灶。在HRD细胞(即brca1 /2缺陷)中,这导致DNA复制不足,当在有丝分裂过程中繁殖时,导致染色体断裂和细胞死亡。我们的发现为基于UNG抑制和尿嘧啶在基因组中的积累的HRD肿瘤的靶向治疗开辟了独特的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
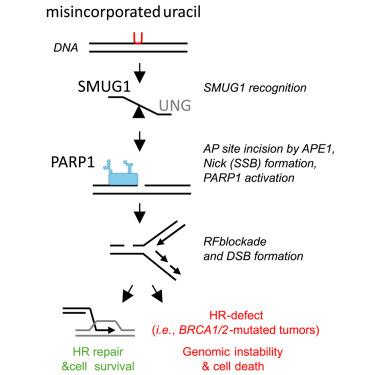
Uracil processing by SMUG1 in the absence of UNG triggers homologous recombination and selectively kills BRCA1/2-deficient tumors
Resistance to poly (ADP-ribose) polymerase (PARP) inhibitors (PARPis) is the major obstacle to their effectiveness in the treatment of homologous recombination (HR)-deficient (HRD) tumors. Hence, developing alternative treatments for HRD tumors is critical. Here, we show that targeting the uracil excision pathway kills HRD tumors, including those with PARPi resistance. We found that the interplay between the two major uracil DNA glycosylases UNG and SMUG1 is regulated by nuclear nicotinamide adenine dinucleotide (NAD+), which maintains UNG at replication forks (RFs) and restrains SMUG1 chromatin binding. In the absence of UNG, SMUG1 retention on chromatin leads to persistent abasic sites, which incision by APE1 results in PARP1 hyperactivation, stalled RFs, and RAD51 foci. In HRD cells (i.e., BRCA1/2-deficient), this leads to under-replicated DNA that, when propagated throughout mitosis, results in chromosome fragmentation and cell death. Our findings open up unique possibilities for targeted therapies for HRD tumors based on UNG inhibition and uracil accumulation in the genome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


