STING介导dnmt3a突变的造血干细胞/祖细胞的自我更新和谱系扭曲
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
DNA 甲基转移酶 3 A(DNMT3A)的体细胞突变经常见于血液恶性肿瘤患者。DNMT3A突变的造血干细胞/祖细胞(HSPCs)显示出更强的自我更新活性和偏斜的品系分化。然而,这些变化背后的分子机制在很大程度上仍未得到探索。在这项研究中,我们发现 Dnmt3a 的缺失会导致 HSPC 中内源性逆转录病毒(ERVs)的上调,进而激活 cGAS-STING 通路并引发这些细胞的炎症反应。遗传和药物抑制 STING 能有效纠正小鼠因缺乏 Dnmt3a 而导致的自我更新活性增强和分化偏斜。值得注意的是,在Dnmt3a-KO; Flt3-ITD AML模型中,靶向STING抑制了急性髓性白血病(AML)的发展,其效果与FDA批准的FLT3-ITD抑制剂AC220相当。患者衍生异种移植(PDX)模型进一步证明,靶向 STING 能有效减轻 DNMT3A 突变 AML 的白血病负担。总之,我们的研究结果凸显了 STING 在 DNMT3A 突变诱导的造血障碍中的关键作用,并提出 STING 是预防 DNMT3A 突变相关白血病进展的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。

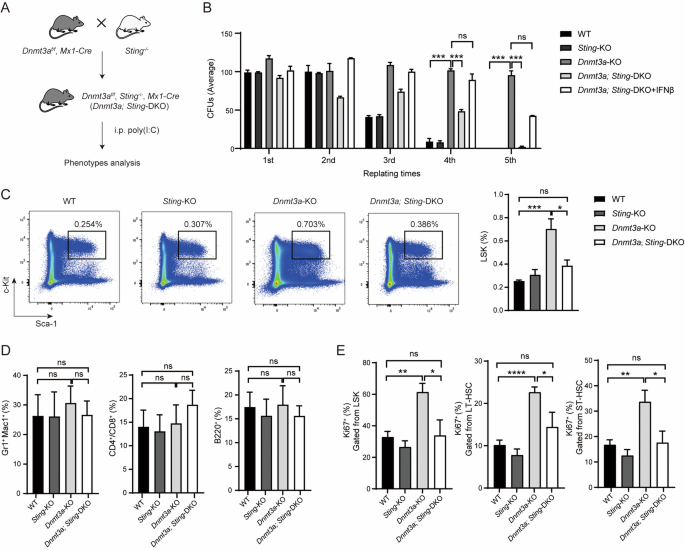
STING mediates increased self-renewal and lineage skewing in DNMT3A-mutated hematopoietic stem/progenitor cells
Somatic mutations in DNA methyltransferase 3 A (DNMT3A) are frequently observed in patients with hematological malignancies. Hematopoietic stem/progenitor cells (HSPCs) with mutated DNMT3A demonstrate increased self-renewal activity and skewed lineage differentiation. However, the molecular mechanisms underlying these changes remain largely unexplored. In this study, we show that Dnmt3a loss leads to the upregulation of endogenous retroviruses (ERVs) in HSPCs, subsequently activating the cGAS-STING pathway and triggering inflammatory responses in these cells. Both genetic and pharmacological inhibition of STING effectively corrects the increased self-renewal activity and differentiation skewing induced by Dnmt3a deficiency in mice. Notably, targeting STING showed inhibited acute myeloid leukemia (AML) development in a Dnmt3a-KO; Flt3-ITD AML model, comparable to AC220, an FDA-approved FLT3-ITD inhibitor. A patient-derived xenograft (PDX) model further demonstrated that targeting STING effectively alleviates the leukemic burden of DNMT3A-mutant AML. Collectively, our findings highlight a critical role for STING in hematopoietic disorders induced by DNMT3A mutations and propose STING as a potential therapeutic target for preventing the progression of DNMT3A mutation-associated leukemia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


