无过渡金属合成C3,5-二官能化氧吲哚衍生物
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02428a
引用次数: 0
摘要
本文提出了一种无过渡金属的一锅催化合成C3, 5-二取代吲哚的新方法。该工艺具有官能团耐受性好、反应条件温和、原子经济性高等特点。此外,该方案为合成具有潜在生物活性的新颖复杂的氧吲哚衍生物提供了一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
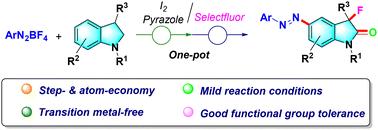
Transition-metal-free synthesis of C3,5-difunctionalized oxindole derivatives†
Herein, a new one-pot transition-metal-free catalytic method for the synthesis of C3,5-disubstituted oxindoles has been developed. The protocol features good functional group tolerance, mild reaction conditions and atom economy. In addition, this protocol provides a platform for the synthesis of novel and complex oxindole derivatives with potential for biological activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


