可见光下通过氢原子转移介导的烯烃吡啶烷基化反应
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d5qo00085h
引用次数: 0
摘要
我们开发了一种可见光介导的合成方案,用于烯烃的三组分吡啶硅烷化反应,以获得 β-吡啶硅酮。通过缺电子的 N-甲氧基吡啶鎓盐和碱之间形成的 EDA 复合物,原位生成的甲氧基自由基启动了反应级联。使用氢硅烷作为硅源,通过硅烷 Si-H 键和甲氧基之间的氢原子转移过程来获得硅基的反应进展顺利。该方案具有无外源光催化剂条件和高原子经济性的特点,从而为制备具有良好官能团兼容性的含硅基和吡啶基化合物提供了一种强大的合成方法。因此,这种方法有望在合成化学和药物发现领域得到应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
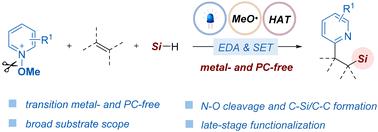
Visible light-mediated pyridyl silylation of olefins through hydrogen atom transfer†
A visible light-mediated synthesis protocol for the three-component pyridyl silylation of olefins to access β-pyridyl silicons has been developed. The reaction cascade is initiated by an in situ generated methoxy radical enabled by EDA complexes formed between the electron-deficient N-methoxypyridinium salts and a base. The reaction using hydrosilane as the silicon source to access the silyl radical proceeds well via a hydrogen atom transfer process between a silane Si–H bond and the methoxy radical. This protocol features exogenous photocatalyst-free conditions and high atom economy, thereby providing a powerful synthon for preparing silyl- and pyridyl-containing compounds with excellent functional group compatibility. Therefore, it is expected that this method will find applications in synthetic chemistry and drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


