实时同位素特征定向谱法发现半胱氨酸羧基烷基化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
数据依赖获取(Data-dependent acquisition, DDA)在散弹枪蛋白质组学中得到了广泛的应用。然而,受质谱(MS)仪器扫描速度的限制,DDA直接检测低丰度肽仍然是一个挑战。在此,我们开发了一种实时靶向质谱数据采集方法“isoSTAR”,该方法在全质谱扫描阶段通过其独特的同位素特征识别目标肽,并立即对其进行靶向质谱扫描。与传统的质谱获取方法相比,该方法在识别低丰度目标肽方面的灵敏度有了显著提高。利用该方法,我们在脂肪酸代谢过程中发现了半胱氨酸的一系列羧基烷基化,并利用合成肽标准验证了它们的修饰结构。我们设想isoSTAR将成为一个强大而通用的工具,以增强霰弹枪蛋白质组学在分析蛋白质中心修饰方面的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
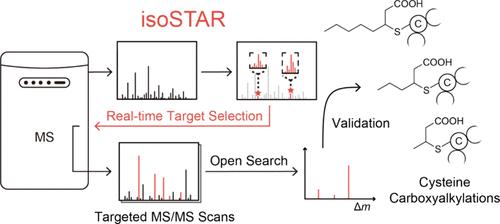
Discovery of Cysteine Carboxyalkylations by Real-Time Isotopic Signature Targeted Profiling
Data-dependent acquisition (DDA) is widely applied in shotgun proteomics. However, restricted by the scanning speed of mass spectrometry (MS) instruments, it remains challenging for DDA to directly detect peptides with low abundance. Herein, we developed a real-time targeted MS data acquisition method, “isoSTAR”, which identifies target peptides by their unique isotopic signatures during the stage of full-MS scanning and subjects them to targeted MS/MS scans immediately. The method showed dramatic improvement in sensitivity in identifying target peptides with low abundance compared to traditional MS acquisition methods. Using this method, we discovered a series of carboxyalkylations on cysteines during fatty acid metabolism and verified their modification structures using synthetic peptide standards. We envision that isoSTAR will become a powerful and versatile tool to enhance shotgun proteomics applications in profiling protein-centric modifications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


