六氟异丙醇丙烯酰胺的氢键网络终端选择性异芳基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
六氟异丙醇(HFIP)介导的烯酰胺的末端选择性异芳基化已经通过h键网络激活的底物以稳健的方式完成。该策略的特点是一个级联过程,包括顺序亲核加成和亲电异芳取代,非常适合复杂生物活性分子的后期功能化。研究人员通过多项对照实验、动力学研究、同位素标记实验和hfip -烯酰胺中间加合物的分离来阐明其潜在机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
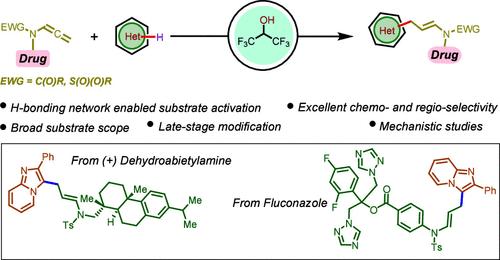
Hydrogen-Bonding Network-Enabled Terminal Selective Heteroarylation of Allenamides in Hexafluoroisopropanol
Hexafluoroisopropanol (HFIP)-mediated terminal selective heteroarylation of allenamides has been accomplished through H-bonding network-enabled substrate activation in a robust fashion. This strategy features a cascade process involving sequential nucleophilic addition followed by electrophilic heteroaromatic substitution and is well suited for late-stage functionalization of complex bioactive molecules. The elucidation of the underlying mechanism was achieved through a comprehensive combination of several control experiments, kinetic studies, isotopic labeling experiments, and the isolation of the HFIP–allenamide intermediate adduct.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


