设计一种嵌段共聚物膜,用于乳酸在水溶液中的选择性运输
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
我们报道了三嵌段共聚物基膜的设计和合成,使乳酸从水溶液中选择性运输。这与聚乳酸的生产有关,聚乳酸是少数具有足够机械强度的生物可降解和生物基聚合物之一,可用于实际应用。端块带正电荷和带负电荷的乳酸反离子。中间嵌段是聚丁二烯(PBD)。由于微相分离,带电块形成输送乳酸的通道。通过交联PBD块来控制膜的机械完整性。通过将膜放置在两个腔室之间,研究了乳酸和水在膜上的运输,一个是含有乳酸水溶液的进料腔,一个是含有纯水的接收腔。乳酸浓度在接收室监测作为一个函数的时间使用电导率,高效液相色谱和核磁共振。采用核磁共振成像方法测量了从接收室到进料室的相应水通量。乳酸透过率和水透过率分别为(1.12±0.05)× 10-8和(8.58±0.75)× 10-9 cm2 s-1。据我们所知,在文献中没有关于乳酸通过任何膜的渗透性的报道。我们的膜的分离因子αLA/water为1.305±0.123,与用于乙醇选择性运输的膜相当,尽管乳酸的分子比乙醇大得多。乳酸在我们的膜中的选择性运输主要受溶解度差异的支配;乳酸在膜中的可溶性是水的18倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
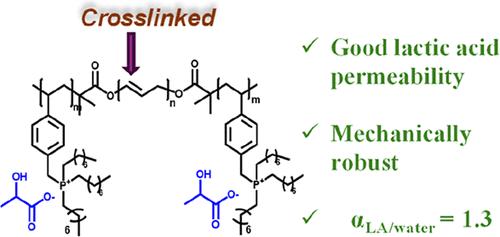
Designing a Block Copolymer Membrane for Selective Transport of Lactic Acid from Aqueous Mixtures
We report the design and synthesis of a triblock copolymer-based membrane for enabling selective transport of lactic acid from aqueous solutions. This is relevant to the production of polylactic acid, one of the few biodegradable and biobased polymers with sufficient mechanical strength for practical applications. The end blocks are positively charged with negatively charged lactate counterions. The middle block is polybutadiene (PBD). Due to microphase separation, the charged blocks form channels for transporting lactic acid. The mechanical integrity of the membrane is controlled by cross-linking the PBD block. Transport of lactic acid and water across the membrane was studied by placing the membrane between two chambers, a feed chamber containing aqueous lactic acid solutions, and a receiving chamber containing pure water. The lactic acid concentration in the receiving chamber was monitored as a function of time using conductivity, HPLC, and NMR. The corresponding flux of water from the receiving chamber to the feed chamber was measured using an NMR-based approach. The lactic acid and water permeabilities through our membrane were (1.12 ± 0.05) × 10–8 and (8.58 ± 0.75) × 10–9 cm2 s–1. To our knowledge, there are no reports of lactic acid permeabilities through any membrane in the literature. The separation factor of our membrane, αLA/water, 1.305 ± 0.123, is comparable to that of membranes used for selective transport of ethanol, despite the fact that lactic acid is a much larger molecule than ethanol. Selective transport of lactic acid in our membrane is governed mainly by differences in solubility; lactic acid is 18 times more soluble in the membrane than water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


