基于化学可回收环烷基取代聚羟基烷酸酯的超韧热塑性弹性体
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚羟基烷酸酯(PHAs)化学再循环制备丙内酯基单体是一个长期存在的挑战,因为它具有较高的环应变和许多不可避免的副反应。在这项贡献中,设计了一种新型α-螺环己基丙内酯(SHPL),即使在催化剂负载为1ppm时也具有高开环聚合反应活性。所得聚(3-羟基-2-螺-环己基丙酸酯)(P3HSHP)具有较高的热稳定性,Td为364℃,Tm为272℃。同时,它可以解聚回SHPL,产率为86%,没有脱羧或消除副产物。值得注意的是,SHPL可以通过与ε-己内酯(CL)的一锅共聚来构建高性能热塑性弹性体(tpe)。特别是,得到的梯度P(CL2000-grad-SHPL500)显示出58.8±4.0 MPa的极限拉伸强度,1959±53%的高拉伸率,600 MJ/m3的创纪录韧性和高弹性恢复(>90%)。SHPL的这种优异性能可以促进新型可持续高性能tpe的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
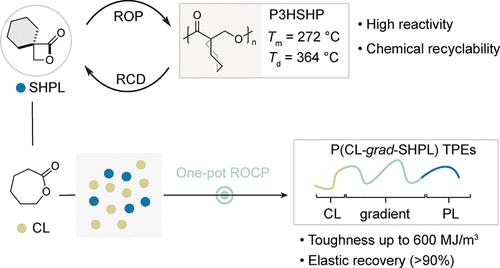
Ultratough Thermoplastic Elastomers Based on Chemically Recyclable Cycloalkyl-Substituted Polyhydroxyalkanoates
It remains a long-standing challenge for chemical recycling of polyhydroxyalkanoates (PHAs) to propiolactone-based monomers due to the high ring strain and many inevitable side reactions. In this contribution, a novel α-spiro-cyclohexyl-propiolactone (SHPL) has been designed with high reactivity toward ring-opening polymerization even at a catalyst loading of <1 ppm. The resulting poly(3-hydroxy-2-spiro-cyclohexylpropionate) (P3HSHP) exhibited high thermal stability with a Td of 364 °C and a high Tm of 272 °C. Meanwhile, it could be depolymerized back to SHPL in 86% yield without decarboxylation or elimination side products. Notably, SHPL could be exploited to construct high-performance thermoplastic elastomers (TPEs) via one-pot copolymerization with ε-caprolactone (CL). Particularly, the resulting gradient P(CL2000-grad-SHPL500) showcased an ultimate tensile strength of 58.8 ± 4.0 MPa, high stretchability of 1959 ± 53%, a record toughness of 600 MJ/m3, and high elastic recovery (>90%). This superior performance of SHPL could advance the development of new sustainable high-performance TPEs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


