非共价键作用下环应变驱动有序异氰化物插入反应合成吡咯衍生物
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02144d
引用次数: 0
摘要
通过环应变驱动和分子内非共价键介导的有序异氰化物插入反应,实现了吡咯衍生物的高效合成。该方法利用前驱体的策略设计来控制反应性,确保选择性和高收率。该策略值得注意的是它创新地利用了α, β-不饱和酮,它同时作为一个导向基团和一个完整的反应底物。此外,我们已经证明了同一底物在碱性或酸性条件下的顺序去叔丁化。通过DFT理论计算进一步证实了这一合理的机理,为我们提出的路线提供了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
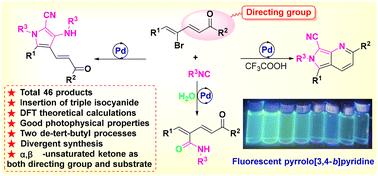
Synthesis of pyrrole derivatives via ordered isocyanide insertion reaction driven by ring strain mediated by non-covalent bond interactions†
An efficient synthesis of pyrrole derivatives is achieved via ordered isocyanide insertion reaction, driven by ring strain and mediated by intramolecular non-covalent bonds. This method harnesses the strategic design of precursors to control reactivity, ensuring selectivity and high yields. The strategy is noteworthy for its innovative utilization of α,β-unsaturated ketone, which concurrently serves as both a directing group and an integral reaction substrate. Additionally, we have demonstrated the sequential de-tert-butylation of the same substrate under alkaline or acidic conditions. The plausible mechanism has been further corroborated through DFT theoretical calculations, providing a solid foundation for our proposed route.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


